Scheme 1.
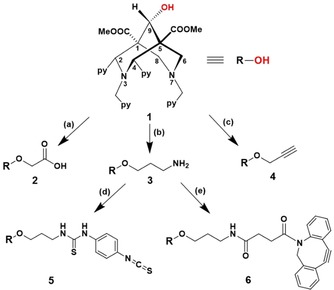
Synthetic approaches to bispidine‐acetic acid 2, bispidine‐amine 3, bispidine‐alkyne 4, bispidine‐isothiocyanate 5 and bispidine‐DBCO 6 by using the bispidine‐9‐ol 1 as the starting compound: (a) THF, sodium hydride (NaH), iodoacetic acid, H2O, 50 °C, 2 h, yield=8.6 %; (b) (i) Dry THF, sodium hydride (NaH), tert‐butyl(3‐bromopropyl)carbamate, sodium hydrogen carbonate (NaHCO3), 50 °C, 20 h, yield=35 %, (ii) TFA/DCM, 1:1 (v/v), RT, 24 h, yield=100 %; (c) Dry THF, sodium hydride (NaH), propargyl bromide, sodium hydrogen carbonate (NaHCO3), 50 °C, 3 h, yield=16 %; (d) DCM, TEA, p‐phenylene diisothiocyanate, RT, 16 h, yield=60 %; (e) DCM, DBCO‐NHS ester, TEA, RT, 2 h, yield=56 %.
