Figure 1.
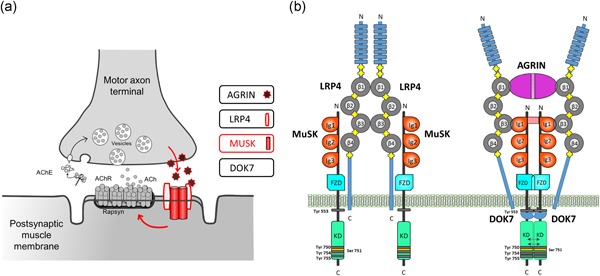
The NMJ and the agrin‐LRP4‐MuSK‐DOK7 pathway. (a) Schematic representation of the NMJ and the agrin‐LRP4‐MuSK‐DOK7 pathway. (b) The binding of agrin to the N‐terminal region of LRP4 induces a conformational change (active state) promoting the binding between LRP4 and MuSK first IgG‐like domain (Zhang, Coldefy, Hubbard, & Burden, 2011). This results in MuSK activation via dimerization and trans‐autophosphorylation of tyrosine residues within the cytoplasmic region (Schlessinger, 2000) through a not fully known mechanism. The increase in the catalytic activity creates active binding sites for other proteins such as DOK7, which amplifies the signal downstream. LRP4 is composed of eight low‐density lipoprotein Class A (LDLa) domains (blue) at the N‐terminus, followed by four YWTD β‐propeller domains (gray) bounded by epidermal growth factor‐like modules (yellow) and a short C‐terminal domain (Springer, 1998). LRP4 self‐associates and interacts with MuSK in the absence of agrin (inactive state), but is not capable of activating MuSK (Kim et al., 2008). This interaction could be important for AChRs prepatterning before innervation (Yang et al., 2001; Yumoto et al., 2012). IgG, immunoglobulin G; LRP, low‐density lipoprotein receptor‐related protein; MuSK, muscle‐specific receptor tyrosine kinase; NMJ, neuromuscular junction
