Figure 3.
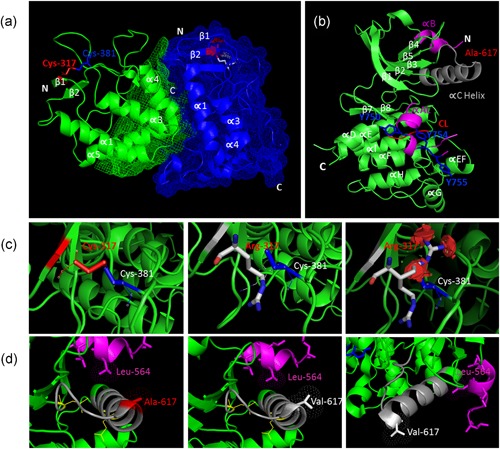
Molecular modeling of MuSK variants. (a) Cartoon representation of MuSK Frizzled‐like domain structure (MMDB ID: 76272) with two copies of the asymmetric dimmer colored in green and blue. Structural elements are labeled in white text according to (Stiegler, Burden, & Hubbard, 2009) and the p.Cys317 residue in red text. (b) Cartoon representation of MuSK KD monomer structure (MMDB ID: 20673). Structural elements are labeled according to (Till et al., 2002). MuSK KD structure is made of β‐strands and α‐helices (green). MuSK activity is regulated by the juxtamembrane domain at the N‐terminus (αB) and the activation loop (αAL; pink). The catalytic loop (CL) is represented in red color. The αC‐helix is colored gray and the p.Ala617 residue is labeled in red. The tyrosine residues within the activation loop (Tyr‐750/754/755) are shown in stick representation (blue). The crystal structure of the MuSK KD after dimerization is not known. (c) The residue p.Cys317 forms a disulfide bond with p.Cys381. Modeling of p.C317R shows how the substitution of cysteine to an arginine result in the disruption of an essential disulfide bond leading to multiple clashes with the neighboring residues. (d) The p.Ala617 residue (red color) is located in the αC‐helix at the N‐terminal lobe of the MuSK KD. Using the crystal structure by (Till et al., 2002), the substitution of alanine to valine does not result in changes in distance and/or a clash with other residues. KD, kinase domain; MuSK, muscle‐specific receptor tyrosine kinase
