Figure 8.
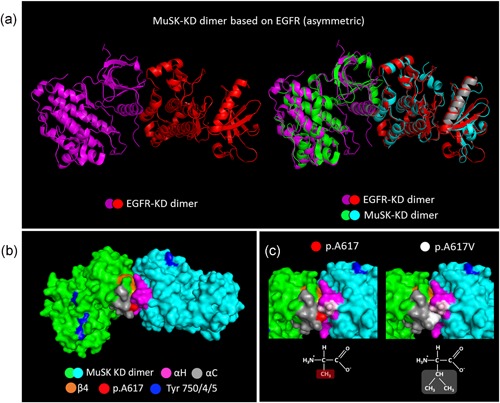
Molecular modeling of MuSK KD dimerization based on the EGFR‐KD dimer structure. (a) MuSK KD asymmetric dimer built by homology modeling based on the crystal structure of the EGFR‐KD activated dimer (MMDB ID: 40024). (b) Surface representation of MuSK KD dimer (green and cyan) based on the EGFR dimer. In the proposed structure, MuSK KDs, colored green and cyan, form an asymmetric dimer with the H‐helix (pink) of one monomer binding to αC‐helix (gray) and β4 sheet (orange) from the contralateral monomer. The p.Ala617 residue (red) is located within the αC‐helix at the dimerization interface. The tyrosines within the activation loop (Tyr‐750/754/755) are in blue. (c) The figure shows how the p.Ala617Val substitution modifies the dimerization interface increasing the hydrophobic interface for the docking of the contralateral MuSK KD. In this model, the p.Ala617 residue (red) interacts with the p.Ala842 and p.Asp843 residues (light purple) from the H‐helix (purple) at the dimerization interface. EGFR, epidermal growth factor receptor; KD, kinase domain; MuSK, muscle‐specific receptor tyrosine kinase
