Historically, anticancer drugs were employed due to their rather unspecific cytotoxic effect on proliferating cells of any kind including malignant cells (for therapeutic cytoreduction) and normal cells (at the cost of major side effects). In recent times, more specific agents, dubbed ‘targeted’ agents have been developed to specifically inhibit enzymes that are required for cancer cells to strive. Although the overall logic for developing cytotoxicants and targeted agents is cancer cell-centric (in the sense that cancer is viewed as a merely cell-autonomous disease), it appears that most if not all anticancer drugs that are clinically successful are actually stressing and killing cancer cells in a way that they elicit an immune response against tumor-associated antigens.1 Hence, it is “immunogenic cell death” (ICD) and the consequent immune attack against residual cancer cells that explain the capacity of anticancer drugs to induce (occasional) long-lasting remissions or to elicit disease stabilization beyond therapeutic discontinuation.2 Importantly, it appears that not all anticancer drugs are equal in their capacity to induce ICD: many kill tumor cells in an immunologically silent fashion, only some elicit full-blown ICD thanks to their capacity to induce cellular stress responses such as autophagy and focused endoplasmic reticulum stress involving the phosphorylation of eIF2alpha. Logically, the drugs that fall into this latter category, ICD inducers, can be advantageously combined with immunotherapies targeting immune checkpoints such as CTLA-4, PD-1 and PD-L1.3
For this reason, it is important to understand which old and new anticancer drugs are efficient ICD inducers. In principal there are two strategies to identify such drugs, namely, (i) systematic screening or (ii) a procedure that we could call “illustrated serendipity” (Figure 1).
Figure 1.
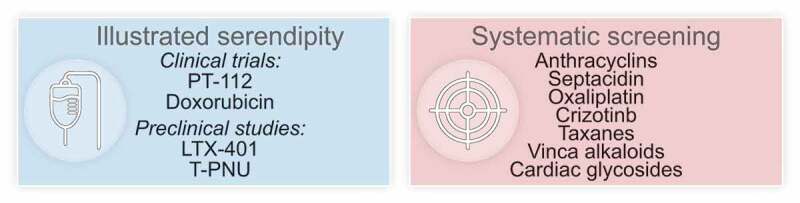
Immunogenic cell death inducer discovery. Drugs that are endowed with the capacity to elicit immunogenic cell death (ICD) can be advantageously combined with immune checkpoint blockade. Thus, the identification of ICD-inducing agents can be driven by “illustrated serendipity”, which is based on findings from clinical (and preclinical) studies depicting improved outcome when the candidate ICD inducer is combined with immune checkpoint blockade (ICB). Alternatively, systems biology approaches can be employed, that predict (based on physical and chemical drug properties) and measure the emission of ICD hallmarks as an indication for the capacity to elicit anticancer immunity. Prospective ICD inducers can be further validated and combined with ICB in immunocompetent animal models.
Systematic screening of compound collections (such as the library of all FDA-approved drugs, the National Cancer Institute panel of cytotoxic agents, a collection of tyrosine kinase inhibitors, etc.)4 has led to the identification of ICD inducers that are in clinical use for cancer treatment (such as anthracyclines, crizotinib, oxaliplatin, taxanes, vinca-alkaloids…),5 for cancer-unrelated diseases (cardiac glycosides)6 or are in preclinical evaluation (such as septacidin).7 These agents kill cancer cells in vitro in a way that the resulting dying/dead cell preparation can be used as a vaccine for eliciting protective anticancer immune responses in vivo, in mice.8 Moreover, these agents mediate tumor growth-reducing effects that are far more efficient in immunocompetent than in immunodeficient mice, meaning that their therapeutic efficacy relies on the immune system.8 Importantly, whenever they have been combined with immune checkpoint inhibitors, they turned out to mediate at least additive, often synergistic effects.9
The method of “illustrated serendipity” is based on clinical (and preclinical) data suggesting advantageous interactions between the candidate ICD inducers and immune checkpoint blockade. Using this approach, Yamazaki et al. recently discovered that the novel platinum-based compound R,R-1,2 cyclohexanediamine-pyrophosphato-platinum(II) (PT-112) can induce ICD. Indeed, prior clinical reports suggested that PT-112 could synergize with PD-L1 targeting immunotherapy,10,11 stimulating the curiosity of this group of researchers with respect to potential ICD-inducing effects of PT-112. In accord with the authors’ suspicion, PT-112 could induce several of the hallmark of ICD when added to cancer cells in vitro: calreticulin exposure on the cell surface, release of ATP from the cytoplasm, and liberation of high mobility group B1 (HMGB1) protein from the cells into the supernatant. Moreover, cells killed with PT-112 elicited a protective immune response in vivo, and tumors treated with PT-112 locally could enable at least some degree of systemic disease control in abscopal models.12 Most importantly, in vivo, in mice, PT-112 sensitized tumors to subsequent treatment with PD-1 blocking antibodies, strongly supporting the rather anecdotic clinical evidence at the preclinical level. Finally, PT-112-treated tumor exhibited signs of improved local immune control with a major increase in the ratio of cytotoxic T lymphocytes over regulatory T cells that was particularly strong when PT-112 was combined with PD-1 blockade.12
Altogether, these findings suggest the possibility that anticancer agents that favorably interact with PD-1/PD-L1-targeting immunotherapy usually act as ICD inducers. Future studies should address this conjecture that, if true, would streamline mode of action studies from a serendipitous to a strongly hypothesis-driven strategy. Most cancer patients impatiently expect therapeutic solutions, and it appears urgent to (in)validate this rationale in future research.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de lRecherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Inserm Transfert; Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Disclosure of potential conflicts of interest
OK and GK are scientific co-founders of Samsara Therapeutics.
References
- 1.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G.. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–2. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 2.Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17:49–64. doi: 10.1038/s41571-019-0272-7. [DOI] [PubMed] [Google Scholar]
- 3.Kepp O, Zitvogel L, Kroemer G. Clinical evidence that immunogenic cell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunology. 2019;8:e1637188. doi: 10.1080/2162402X.2019.1637188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kepp O, Sauvat A, Leduc M, Forveille S, Liu P, Zhao L, Bezu L, Xie W, Zitvogel L, Kroemer G, et al. A fluorescent biosensor-based platform for the discovery of immunogenic cancer cell death inducers. Oncoimmunology. 2019;8:1606665. doi: 10.1080/2162402X.2019.1606665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10:1486. doi: 10.1038/s41467-019-09415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra199. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 7.Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, et al. Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology. 2014;3:e28473. doi: 10.4161/onci.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Zhao L, Kepp O, Kroemer GC. - a tyrosine kinase inhibitor that stimulates immunogenic cell death. Oncoimmunology. 2019;8:1596652. doi: 10.1080/2162402X.2019.1596652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17:854–855. doi: 10.1038/nrd.2018.210. [DOI] [PubMed] [Google Scholar]
- 11.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10:eaat7807. doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki T, Buque A, Ames TD, Galluzzi L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. OncoImmunology. 2020;9:1721810. doi: 10.1080/2162402X.2020.1721810. [DOI] [PMC free article] [PubMed] [Google Scholar]


