Abstract
Background:
Both enhanced recovery and anesthesia literature recommend multimodal perioperative analgesia to hasten recovery, prevent adverse events, and reduce opioid use after surgery. However, adherence to, and outcomes of, these recommendations are unknown. We sought to characterize use of multimodal analgesia and its association with length of stay after colectomy.
Materials and Methods:
Within a statewide, 72-hospital collaborative quality initiative, we evaluated postoperative analgesia regimens among adult patients undergoing elective colectomy between 2012 and 2015. We used logistic regression to identify factors associated with the use of multimodal analgesia, and performed multivariable linear regression to evaluate its association with postoperative length of stay (LOS).
Results:
Among 7265 patients who underwent elective colectomy in the study period, 4660 (64.1%) received multimodal analgesia, 2405 (33.1%) received opioids alone, and 200 (2.8%) received one non-opioid pain medication alone. Multimodal analgesia was independently associated with shorter postoperative LOS, compared with opioids alone (5.60 days [95% CI 5.38 – 5.81] versus 5.96 days [5.68–6.24], p=0.016)
Conclusions:
Multimodal analgesia is associated with shorter LOS, yet one third of patients statewide received opioids alone after colectomy. As surgeons increasingly focus on our role in the opioid crisis, particularly in post-discharge opioid prescribing, we must also focus on inpatient postoperative pain management to limit opioid exposure. At the hospital level, this may have the added benefit of decreasing LOS and hastening recovery.
Keywords: multimodal analgesia, length of stay, colectomy, enhanced recovery, analgesia, opioid
INTRODUCTION
Surgeons and hospitals have faced increasing pressure to decrease length of stay (LOS) after surgery, both for the benefit of patients and their recovery, and also to improve hospital throughput and efficiency in the setting of prospective payment for hospitalization. A variety of measures have been employed to reduce LOS in alimentary tract surgery, including enhanced recovery protocols (ERP),1–3 limiting intravenous fluids,4,5 and alvimopan.6,7 Multimodal analgesia is a key piece of enhanced recovery protocols and may improve mobility, prevent opioid-induced ileus, and hasten the transition to oral regimens.8 Multimodal analgesia regimens using nonsteroidal anti-inflammatory drugs (NSAIDS), acetaminophen, epidurals, and nerve blocks can decrease opioid consumption while preserving pain control.9–14 Perioperative pain control guidelines recommend limiting opioids15 and have encouraged multimodal analgesia for years,16 but there is even greater interest in multimodal regimens as new evidence arises implicating perioperative opioids in new, persistent opioid use.17–19
Improving pain control using multimodal analgesia may accelerate post-operative recovery and shorten hospital stay, but real-world use of multimodal analgesia and its effect on hospital stay is understudied. A Cochrane review of randomized controlled trials (RCTs) comparing epidural analgesia versus intravenous opioids found no difference in LOS,20 while a meta-analysis of ERP RCTs found shorter LOS, with 5 of the 6 studies using epidural analgesia and avoiding systemic morphine use.21 However, in real-world practice, as opposed to RCTs, adherence to ERP and multimodal analgesia recommendations is often imperfect.3,22 Even highly focused programs attain only a 76% compliance rate with the ERP.23 It is unknown how commonly surgeons provide multimodal analgesia, and whether it achieves the goal of shorter postoperative length of stay.
In order to evaluate use and outcomes of multimodal analgesia, we evaluated colorectal resections performed within a statewide surgical quality collaborative collecting specific pain management data and validated clinical parameters in the perioperative period. We chose to focus on colectomy because it is a common major operation, performed in a wide variety of hospitals, and has been included in many of the earliest ERP protocols, both within our state and more broadly.24 Therefore, we sought to characterize the post-operative medication regimen and the rates of multimodal analgesia use. We also aimed to identify whether there is an effect of multimodal analgesia use on length of stay after surgery for patients undergoing elective colectomy. We hypothesized that multimodal analgesia would be associated with shorter LOS.
MATERIALS AND METHODS:
We performed a retrospective cohort study using clinical registry data from the Michigan Surgical Quality Collaborative (MSQC), a large, statewide surgical registry that includes every major surgical hospital in the state, with a focus on sampling for colectomy. Data is manually abstracted from electronic and paper records by trained nurses and the registry is audited regularly for validity. Collected data include patient characteristics, perioperative processes of care, and postoperative clinical outcomes. The current study was performed with University of Michigan IRB approval HUM00090549.
Patients and procedures
We included patients age 18 and older, undergoing elective colectomy (CPT 44140, 44141, 44143, 44144–47, 44160, 44204–08) between July 2012 and October 2015. After October 2015, data abstraction of pain regimen was no longer mandatory in MSQC. Patients were excluded from the sample if there was missing data for all pain medication variables (n=13). Patients with missing data were excluded from the regression (hospital bed size and teaching status, n=49; insurance status, n=40; totaling 1.3% of the cohort).
Measures
Pain medications captured in the registry included intravenous and oral opioids, acetaminophen, non-steroidal anti-inflammatories (NSAIDs), epidural analgesia, and local analgesia, all identified for the first 24 hours post-operatively.
The primary outcome variable was length of stay. In order to reduce the influence of outliers, but still maintain the effect of prolonged length of stay, we winsorized LOS to 30 days, which assigned a value of 30 days to any LOS beyond 30 days. The primary predictor was receiving multimodal analgesia, defined as a dichotomous variable (0 = opioid alone, 1 = multimodal analgesia: one or more non-opioid medications ± opioid) ordered in the first 24 hours postoperatively. A minority of the study population received a single-agent non-opioid pain regimen (epidural, acetaminophen, NSAID, or local anesthesia). As the patients comprising the single-agent non-opioid cohort were primarily laparoscopic operations and more likely to be treated in smaller hospitals, they were not comparable to the other two cohorts. Accordingly, multimodal analgesia was used for the full analysis and all three pain regimens (multimodal analgesia, opioid alone, and single-agent non-opioid) were used in sensitivity analyses.
Statistical Analyses
We performed bivariate analyses to identify factors associated with pain regimen and length of stay. To evaluate receipt of multimodal analgesia, we used bivariate factors with p value ≤0.1 and a priori clinically defined factors (post-operative complications, comorbid conditions, hospital teaching status, patient insurance, surgical approach) in a multivariable logistic regression to identify factors independently associated with receipt of multimodal analgesia. To evaluate LOS, again informed by the results of bivariate analyses, we included covariates with p value ≤0.1 in a multivariable linear regression to identify an association between pain regimen (opioids alone versus multimodal analgesia) and LOS. For LOS analyses, we also evaluated other models, including Poisson and negative binomial regression, as well as a linear multilevel model with random hospital and surgeon effects. These models identified the same statistically significant predictors and similar effect sizes. For ease of understanding and interpreting the data, we used multivariable linear regression for the primary analysis and we used robust standard errors to correct for cluster effects at the hospital level. In both multimodal analgesia and LOS analyses, we controlled for patient age, race/ethnicity, American Society of Anesthesiologists (ASA) class, postoperative complications, surgical approach (open versus minimally invasive), hospital bed size, hospital teaching status, and patient insurance status. We did not include any a priori defined interaction terms in the primary analysis. There was no evidence for multicollinearity using the variance inflation factor. As a secondary analysis, we evaluated a variety of interaction terms, representing specific combinations of medications present in at least 1% of patients (to allow for reliable effect estimation), in a multivariable linear regression.
All statistical tests were two-sided and a p value of <0.05 was used as significance threshold. All analyses were conducted using Stata 15.1, StataCorp, College Station, TX.
RESULTS
Patient Characteristics
The cohort of 7265 patients had a mean age of 62.5 (SD 13.8) years and a range of 18 to 89 years. Over half of the cohort was female and the vast majority (84.6%) was white. When separated by pain regimen, the three groups had statistically significant differences by age, race, comorbidities, surgical approach (open vs. laparoscopic), insurance status, and hospital characteristics (Table 1).
Table 1:
Patient characteristics, by pain regimen.
| Characteristics | Opioids Alone N (%) | Multimodal N (%) | Opioid-Free, Single Agent N (%) | p-value | |
|---|---|---|---|---|---|
| 2405 (33.1) | 4660 (64.1) | 200 (2.8) | - | ||
| <45 | 181 (7.5) | 519 (11.1) | 13 (6.5) | <0.001 | |
| 45 to 64 | 962 (40.0) | 2116 (45.4) | 90 (45.0) | ||
| ≥ 65 | 1262 (52.5) | 2025 (43.5) | 97 (48.5) | ||
| Female | 1295 (53.9) | 2551 (54.7) | 117 (58.5) | 0.41 | |
| White | 1920 (79.8) | 4039 (86.7) | 183 (91.5) | <0.001 | |
| Black | 362 (15.1) | 424 (9.1) | 10 (5.0) | ||
| Other | 123 (5.1) | 195 (4.2) | 7 (3.5) | ||
| Obese (BMI >30) | 895 (37.4) | 1740 (37.5) | 75 (37.5) | 1.0 | |
| ASA Class: 3–4 | 1396 (58.1) | 2358 (50.6) | 111 (55.5) | <0.001 | |
| Tobacco Use | 471 (19.6) | 1025 (22.0) | 37 (18.5) | 0.041 | |
| Alcohol Use (>2 drinks/day) | 78 (3.2) | 152 (3.3) | 7 (3.5) | 0.98 | |
| Diabetes | 535 (22.3) | 855 (18.4) | 31 (15.5) | <0.001 | |
| Chronic Obstructive Pulmonary Disease | 229 (9.5) | 417 (9.0) | 18 (9.0) | 0.73 | |
| Hypertension | 1395 (58.0) | 2471 (53.0) | 112 (56.0) | <0.001 | |
| Peripheral Vascular Disease | 74 (3.1) | 102 (2.2) | 8 (4.0) | 0.032 | |
| Chronic Steroid Use | 132 (5.5) | 255 (5.5) | 6 (3.0) | 0.31 | |
| Open | 817 (34.0) | 1671 (35.9) | 36 (18.0) | <0.001 | |
| Laparoscopic | 1588 (66.0) | 2989 (64.1) | 164 (82.0) | ||
| Partial Colectomy | 2326 (96.7) | 4468 (96.0) | 195 (97.5) | 0.37 | |
| Total Colectomy | 35 (1.5) | 86 (1.9) | 3 (1.5) | ||
| Coloproctectomy | 44 (1.8) | 106 (2.3) | 2 (1.0) | ||
| Medicare | 1212 (50.7) | 1922 (41.5) | 92 (46.0) | <0.001 | |
| Medicaid | 83 (3.5) | 163 (3.5) | 2 (1.0) | ||
| Private | 1029 (43.1) | 2299 (49.6) | 100 (50.0) | ||
| Other | 66 (2.8) | 251 (5.4) | 6 (3.0) | ||
| Hospital size >=500 beds | 675 (28.3) | 1160 (25.1) | 54 (27.0) | 0.003 | |
| 300–499 beds | 986 (41.3) | 2031 (43.9) | 69 (34.5) | ||
| <300 beds | 727 (30.4) | 1437 (31.1) | 77 (38.5) | ||
| Academic Hospital | 616 (25.8) | 972 (21.0) | 52 (26.0) | <0.001 | |
| Postoperative Complication | 347 (14.4) | 633 (13.6) | 27 (13.5) | 0.62 | |
| Readmission | 262 (10.9) | 454 (9.7) | 20 (10.0) | 0.31 | |
| Intravenous opioid | 2389 (99.3) | 4176 (89.6) | 0 | <0.001 | |
| Oral opioid | 999 (41.6) | 2169 (44.6) | 0 | <0.001 | |
| Acetaminophen | 0 | 2699 (57.9) | 12 (6.0) | <0.001 | |
| Nonsteroidal Anti-inflammatory Drug (NSAID) | 0 | 2546 (54.6) | 14 (7.0) | <0.001 | |
| Epidural | 0 | 1233 (26.5) | 173 (86.5) | <0.001 | |
| Local Anesthetic | 0 | 99 (2.1) | 1 (0.5) | <0.001 | |
Pain regimens
Overall, 6778 patients (93.3%) received an opioid for post-operative pain control, with 2405 (33.1%) of patients receiving opioids alone. Intravenous opioids were the most commonly ordered pain medication (6565 patients, 90.4%), followed by oral opioids (3168, 43.6%), acetaminophen (2711, 37.3%), NSAIDs (2560, 35.3%), epidural analgesia (1406, 19.4%) and local anesthetic (100, 1.4%). Two hundred patients (2.8%) received one class of non-opioid medication alone; of those, 173 (86.5%) received epidural analgesia, 14 (7.0%) an NSAID, 12 (6.0%) acetaminophen, and 1 (0.5%) local anesthetic only (Table 1).
Unadjusted associations to lower rates of receiving multimodal analgesia included oldest patient group (65–84 years), diabetes, hypertension, peripheral vascular disease, ASA class 3 and 4, Medicare insurance, Black race, academic hospital, and >500 beds. In bivariate analysis, readmission rates were similar among the three groups of pain regimens (Table 1). After multivariable adjustment, several characteristics were associated with the receipt of multimodal analgesia. Patients aged 65 – 84 were less likely compared to those in younger age groups (OR 0.82, 95% CI 0.69–0.96, p=0.02), while Black patients were less likely compared to those of other races (OR 0.61 95% CI 0.51–0.73, p<0.001). Patients with ASA class 3–4 (OR 0.88, 95% CI 0.77–1.0, p=0.04) also had a lower likelihood of receiving multimodal analgesia (Table 2). Repeating the analysis adding the 200 patients who received a single-agent non-opioid pain regimen resulted in negligible changes to the effect sizes.
Table 2:
Results of the multivariable logistic regression model to identify associations between patient and clinical characteristics and use of multimodal analgesia.
| Use of Multimodal Pain Therapy | |||
|---|---|---|---|
| Multivariable Logistic Regression | |||
| Covariate | Coefficient | 95% CI | p value |
| Complication | 1.01 | 0.84–1.21 | 0.89 |
| Age - 65–84 years* | 0.82 | 0.69–0.96 | 0.02 |
| Age - ≤44 years* | 1.32 | 1.10–1.57 | 0.002 |
| Race (Black/Other v. White) | 0.61 | 0.51–0.73 | <0.001 |
| ASA3/4 | 0.88 | 0.77–1.00 | 0.04 |
| Hypertension | 1.02 | 0.88–1.17 | 0.84 |
| Peripheral Vascular Disease | 0.78 | 0.55–1.10 | 0.16 |
| Diabetes | 1.00 | 0.89–1.11 | 0.94 |
| Active smoker | 1.06 | 0.91–1.22 | 0.45 |
| Teaching hospital | 0.80 | 0.29–2.17 | 0.67 |
| Minimally invasive approach | 1.00 | 0.80–1.24 | 0.99 |
| Hospital bedsize <300** | 1.02 | 0.42–2.49 | 0.97 |
| Hospital bedsize 300–499** | 1.09 | 0.44–2.69 | 0.85 |
| Insurance - Medicaid*** | 1.06 | 0.80–1.40 | 0.69 |
| Insurance - Private*** | 1.19 | 1.02–1.40 | 0.03 |
| Insurance - Other*** | 2.05 | 0.77–5.43 | 0.15 |
| Constant | 3.57 | ||
Model was constructed using all covariates from the bivariate analysis with p value <0.1 Number of observations: 6974
Pseudo R2 = 0.019
Comparator is Age 45–64
Comparator is bedsize >=500
Comparator is Medicare
Length of Stay
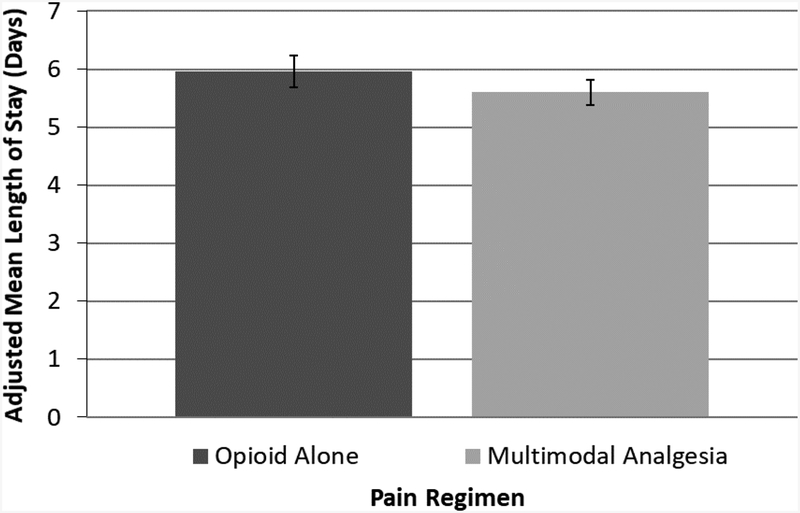
In the overall sample, prior to winsorization, LOS ranged from 1 to 69 days with mean 5.77 days (SD 4.27). After winsorizing LOS to 30 days, mean LOS was 5.74 days (SD 4.03 days). Unadjusted LOS was 5.52 days (SD 3.91) days for multimodal analgesia, versus 6.12 days (SD 4.23) for opioid alone, and 6.53 (SD 3.99) days for single-agent, non-opioid analgesia. After controlling for patient and hospital factors (Table 3), receipt of multimodal analgesia was associated with a 0.36-day shorter length of stay than opioid alone (5.60 days [95% CI 5.38 – 5.81] versus 5.96 days [5.68–6.24], p=0.016) (Figure 1).
Table 3:
Results of the multivariable linear regression model to identify the association between multimodal analgesia and length of stay.
| Length of Stay | |||
|---|---|---|---|
| Multivariable Linear Regression | |||
| Covariate | Coefficient | 95% CI | p value |
| Multimodal Pain Therapy | −0.36 | −0.65 – −0.07 | 0.015 |
| Complication | 4.30 | 3.78–4.83 | <0.001 |
| Age (continuous) | 0.007 | −0.004–0.019 | 0.22 |
| Race (Black/Other v. White) | 0.70 | 0.29–1.11 | 0.001 |
| ASA3/4 | 0.83 | 0.60–1.06 | <0.001 |
| Hypertension | −0.013 | −0.19–0.16 | 0.88 |
| Peripheral Vascular Disease | 0.55 | −0.40–1.50 | 0.25 |
| Diabetes | −0.14 | −0.37–0.088 | 0.22 |
| Active smoker | 0.078 | −0.17–0.32 | 0.54 |
| Teaching hospital | 0.12 | −0.47–0.71 | 0.68 |
| Minimally invasive approach | 0.56 | 0.29–0.82 | <0.001 |
| Hospital bedsize <300* | −0.028 | −0.66–0.60 | 0.93 |
| Hospital bedsize 300–499* | −0.18 | −0.76–0.38 | 0.52 |
| Insurance - Medicaid** | 0.19 | −0.41–0.79 | 0.53 |
| Insurance - Private** | −0.53 | −0.74– −0.32 | <0.001 |
| Insurance - Other** | −0.57 | −1.28–0.14 | 0.11 |
| Constant | 4.73 | ||
Model was constructed using all covariates from the bivariate analysis with p value <0.1 Number of observations: 6974
R2 = 0.19
Comparator is bedsize >=500
Comparator is Medicare
Figure 1:
Adjusted mean length of stay after elective colectomy, by pain regimen. p=0.03
Next, we evaluated whether particular pain medication classes or combinations thereof had a greater effect on LOS. After including pain medication classes individually as dichotomous variables, we found that NSAIDs were associated with a decrease in LOS of 0.62 days (p<0.001) and oral opioids a decrease of 0.90 days (p<0.001), whereas both epidural/spinal analgesia and intravenous opioids were associated with an increase in LOS (0.88 days and 0.73 days, respectively, both p<0.001). There was no association between acetaminophen and LOS. When evaluating for specific combinations of pain medications through interaction terms, among combinations present in at least 1% of patients, there were no statistically significant effects attributable to any specific combinations.
DISCUSSION
Almost all patients received an opioid after elective colectomy and a third received opioids alone in this statewide cohort. Postoperative pain control guidelines have recommended multimodal approaches for many years,15 so this finding suggests that there is substantial opportunity for improvement in perioperative analgesia regimens in Michigan hospitals. Older patients and those with higher ASA classification were less likely to receive multimodal analgesia, perhaps due to comorbid conditions limiting candidacy for acetaminophen or NSAIDs. Black patients also had lower likelihood of receiving multimodal medication, which could be a provider-level factor in practice patterns and practice site, reflection of other health status, or unequal treatment.25
We also found that multimodal analgesia was associated with decreased length of stay. While this difference was small—approximately one third of a day—it may represent the independent influence of these pain regimens, that, combined with other ERP elements and aggregated over many patients, can improve patient throughput and resource utilization in hospitals. It is likely that improved pain management from these regimens contributes to the LOS-shortening effects of ERPs.20,26,27 A meta-analysis analyzing pain control regimens in colorectal surgery with ERP did not find an effect on LOS, however the included studies were primarily head-to-head pain modality RCTs of epidurals, IV lidocaine, wound infusions, and local blocks, which were used only in a minority of cases in our study.28 Rather, real-life regimens of non-opioid medications in combination with opioids may provide effective pain control, reduce overall opioid consumption with the potential to reduce ileus, while allowing patients to ambulate and move towards discharge.
Pain control is a key element of perioperative care and the trend toward referring to pain as “the fifth vital sign” particularly increased attention to analgesia over the last decade.29,30 However, with the ongoing opioid crisis, there is growing awareness of surgical procedures as inciting episodes for new, persistent opioid use.31 In addition, unused prescriptions’ potential for diversion and misuse have increased scrutiny on pain management during the surgical episode.32 Prescriptions after discharge are the primary focus of this scrutiny,33 though calls for reform include setting patient expectations before surgery and during the hospitalization, as well as leveraging the expertise of the multidisciplinary team.17,34 We demonstrate that surgeons still are not providing optimal multimodal pain management, and perhaps that we rely too heavily on opioids during the inpatient stay. Increasing multimodal pain management during hospitalization may contribute to limiting post-discharge opioid use.
This study has several limitations. First, using robust registry data we were able to identify pain medication ordered in the first 24 hours after surgery for a large cohort of patients undergoing elective colectomy, but this does not necessarily reflect administration of these medications nor is actual opioid consumption captured. In addition, some of the opioid-only patients may have had non-opioid analgesics ordered beyond the first 24 hours. However, orders are indicative of surgeon practice and reflect pain management options available to patients. Additionally, the registry data did not capture discharge with an opioid prescription, which is a clinically-relevant outcome of inpatient pain regimen. Second, during the study time period, data on transversus abdominis plane (TAP) blocks, gabapentin, and ketamine use were not collected, though use of those modalities is increasingly referenced in guidelines and reviews.15,19,35 A third limitation is confounding by indication – there may be factors associated with the decision to order multimodal analgesia for a patient that are also associated with factors influencing length of stay. For example, some providers may preferentially use ERPs, which may promote both multimodal analgesia and early discharge, whereas a provider treating older patients or patients in ASA class 3 or 4 may be reticent to prescribe NSAIDs with impaired renal function and also be slower to discharge such patients.36 To account for patient factors, we performed multivariable regression using the measured and known variables, however there may be other pain management-related variables that are unmeasured, particularly alternative pain management techniques not captured in the registry: music therapy,37,38 deep breathing, massage, and relaxation techniques.39 Fourth, return of bowel function is not captured by the registry and may limit prescribing of oral medications postoperatively. The registry, however, captures intravenous non-opioid medication including acetaminophen and ketorolac. In addition, many enhanced recovery protocols initiate clear liquids within the first postoperative day, prior to return of bowel function. Finally, it may not be that a change in post-operative pain regimens will independently affect length of stay, but rather that multimodal pain therapy is a critical part of a set of interventions that result in faster recovery.
No patient should experience poorly controlled pain, and multimodal analgesia is an easy step for surgeons to take to control pain while helping patients limit opioid exposure and the associated downstream risks. As surgeons increasingly focus on our role in the opioid crisis, particularly in post-discharge opioid prescribing, we must also focus on inpatient pain management to limit opioid exposure. At the hospital level, this may have the added benefit of decreasing LOS and increasing throughput. Adequately treating pain while limiting opioid consumption should be the end goal. This can be improved through better adherence to guidelines, and through increased education, and accountability in regional quality collaboratives.
ACKNOWLEDGEMENTS
Funding: ADR is supported by the Agency for Healthcare Research and Quality by T32HS000053 and the National Clinician Scholars Program; JVV is supported by the National Institutes of Health Ruth L. Kirschstein National Research Service Award 1F32DK115340-01A1; SER is supported by the National Institute on Aging 1K08AG047252-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Competing Interests: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
REFERENCES
- 1.Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N, Zurich Fast Track Study G. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009;136:842–847. [DOI] [PubMed] [Google Scholar]
- 2.Miller TE, Thacker JK, White WD, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–1061. [DOI] [PubMed] [Google Scholar]
- 3.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011:Cd007635. [DOI] [PubMed] [Google Scholar]
- 4.Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–826. [DOI] [PubMed] [Google Scholar]
- 5.Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;114:640–651. [DOI] [PubMed] [Google Scholar]
- 6.Delaney CP, Wolff BG, Viscusi ER, et al. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Ann Surg. 2007;245:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240:728–734; discussion 734–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed J, Lim M, Khan S, McNaught C, Macfie J. Predictors of length of stay in patients having elective colorectal surgery within an enhanced recovery protocol. Int J Surg. 2010;8:628–632. [DOI] [PubMed] [Google Scholar]
- 9.Romsing J, Moiniche S, Mathiesen O, Dahl JB. Reduction of opioid-related adverse events using opioid-sparing analgesia with COX-2 inhibitors lacks documentation: a systematic review. Acta Anaesthesiol Scand. 2005;49:133–142. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson BJ, Doane MA. Transversus Abdominis Plane Catheters for Analgesia Following Abdominal Surgery in Adults. Reg Anesth Pain Med. 2018;43:5–13. [DOI] [PubMed] [Google Scholar]
- 11.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104:1545–1556, table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Johns N, O’Neill S, Ventham NT, Barron F, Brady RR, Daniel T. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorectal Dis. 2012;14:e635–642. [DOI] [PubMed] [Google Scholar]
- 13.Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106:292–297. [DOI] [PubMed] [Google Scholar]
- 14.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain--a systematic review of randomized controlled trials. Pain. 2006;126:91–101. [DOI] [PubMed] [Google Scholar]
- 15.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Reg Anesth Pain Med, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. [DOI] [PubMed] [Google Scholar]
- 16.Kehlet H Acute pain control and accelerated postoperative surgical recovery. Surg Clin North Am. 1999;79:431–443. [DOI] [PubMed] [Google Scholar]
- 17.Stone AB, Wick EC, Wu CL, Grant MC. The US Opioid Crisis: A Role for Enhanced Recovery After Surgery. Anesth Analg. 2017;125:1803–1805. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Kirksey MA, Duong S, Wu CL. A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. Anesth Analg. 2017;125:1749–1760. [DOI] [PubMed] [Google Scholar]
- 19.Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017;152:691–697. [DOI] [PubMed] [Google Scholar]
- 20.Guay J, Nishimori M, Kopp SL. Epidural Local Anesthetics Versus Opioid-Based Analgesic Regimens for Postoperative Gastrointestinal Paralysis, Vomiting, and Pain After Abdominal Surgery: A Cochrane Review. Anesth Analg. 2016;123:1591–1602. [DOI] [PubMed] [Google Scholar]
- 21.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–440. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed J, Khan S, Gatt M, Kallam R, MacFie J. Compliance with enhanced recovery programmes in elective colorectal surgery. Br J Surg. 2010;97:754–758. [DOI] [PubMed] [Google Scholar]
- 23.ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg. 2015;261:1153–1159. [DOI] [PubMed] [Google Scholar]
- 24.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg. 2008;207:698–704. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10:1187–1204. [DOI] [PubMed] [Google Scholar]
- 26.Baeriswyl M, Zeiter F, Piubellini D, Kirkham KR, Albrecht E. The analgesic efficacy of transverse abdominis plane block versus epidural analgesia: A systematic review with meta-analysis. Medicine (Baltimore). 2018;97:e11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebl JR, Dilger JA, Byer DE, et al. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–517. [PubMed] [Google Scholar]
- 28.Chemali ME, Eslick GD. A Meta-Analysis: Postoperative Pain Management in Colorectal Surgical Patients and the Effects on Length of Stay in an Enhanced Recovery After Surgery (ERAS) Setting. Clin J Pain. 2017;33:87–92. [DOI] [PubMed] [Google Scholar]
- 29.Phillips DM. JCAHO pain management standards are unveiled. Joint Commission on Accreditation of Healthcare Organizations. JAMA. 2000;284:428–429. [DOI] [PubMed] [Google Scholar]
- 30.McCaffery M, Pasero CL. Pain ratings: the fifth vital sign. Am J Nurs. 1997;97:15–16. [DOI] [PubMed] [Google Scholar]
- 31.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg. 2017;152:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill MV, McMahon ML, Stucke RS, Barth RJ Jr., Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 34.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic Opioid Dependence in the United States: Are Surgeons the Gatekeepers? Ann Surg. 2017;265:728–730. [DOI] [PubMed] [Google Scholar]
- 35.Simpson JC, Bao X, Agarwala A. Pain Management in Enhanced Recovery after Surgery (ERAS) Protocols. Clin Colon Rectal Surg. 2019;32:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YF, Hemming K, Stevens AJ, Lilford RJ. Secular trends and evaluation of complex interventions: the rising tide phenomenon. BMJ Qual Saf. 2016;25:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krout RE. The effects of single-session music therapy interventions on the observed and self-reported levels of pain control, physical comfort, and relaxation of hospice patients. Am J Hosp Palliat Care. 2001;18:383–390. [DOI] [PubMed] [Google Scholar]
- 38.Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a nonpharmacological pain management tool in modern medicine. Neurosci Biobehav Rev. 2011;35:1989–1999. [DOI] [PubMed] [Google Scholar]
- 39.Bruckenthal P Integrating Nonpharmacologic and Alternative Strategies Into a Comprehensive Management Approach for Older Adults With Pain. Pain Manag Nurs. 2010;11:S23–S31. [DOI] [PubMed] [Google Scholar]