Abstract
BACKGROUND AND PURPOSE:
Transverse sinus stenosis can lead to pseudotumor cerebri syndrome by elevating the cerebral venous pressure. The occipital emissary vein is an inconstant emissary vein that connects the torcular herophili with the suboccipital veins of the external vertebral plexus. This retrospective study compares the prevalence and size of the occipital emissary vein in patients with pseudotumor cerebri syndrome with those in healthy control subjects to determine whether the occipital emissary vein could represent a marker of pseudotumor cerebri syndrome.
MATERIALS AND METHODS:
The cranial venous system of 46 adult patients with pseudotumor cerebri syndrome (group 1) was studied on CT venography images and compared with a group of 92 consecutive adult patients without pseudotumor cerebri syndrome who underwent venous assessment with gadolinium-enhanced 3D-T1 MPRAGE sequences (group 2). The presence of an occipital emissary vein was assessed, and its proximal (intraosseous) and distal (extracranial) maximum diameters were measured and compared between the 2 groups. Seventeen patients who underwent transverse sinus stent placement had their occipital emissary vein diameters measured before and after stent placement.
RESULTS:
Thirty of 46 (65%) patients in group 1 versus 29/92 (31.5%) patients in group 2 had an occipital emissary vein (P < .001). The average proximal and distal occipital emissary vein maximum diameters were significantly larger in group 1 (2.3 versus 1.6 mm, P <.005 and 3.3 versus 2.3 mm, P < .001). The average maximum diameters of the occipital emissary vein for patients who underwent transverse sinus stent placement were larger before stent placement than after stent placement: 2.6 versus 1.8 mm proximally (P < .06) and 3.7 versus 2.6 mm distally (P < .005).
CONCLUSIONS:
Occipital emissary veins are more frequent and larger in patients with pseudotumor cerebri syndrome than in healthy subjects, a finding consistent with their role as collateral venous pathway in transverse sinus stenosis. A prominent occipital emissary vein is an imaging sign that should raise the suspicion of pseudotumor cerebri syndrome.
Emissary veins of the skull base and posterior fossa play an important role in directing cerebral venous blood toward cervical outflow pathways.1,2 Emissary veins include the anterior, posterior, and lateral condylar emissary veins, the mastoid emissary vein, and the occipital emissary vein (OEV).1 The OEV is located at or close to the midline of the occipital squama and connects the torcular herophili or distal superior sagittal sinus to the suboccipital veins that drain into the vertebral artery venous plexus, the deep cervical vein, or both (Fig 1).3 The OEV, when present, may connect with the diploic veins of the occipital and parietal bones. Due to its proximal location, the OEV is the only emissary vein of the skull base/posterior fossa that can provide a derivation pathway for the transverse sinus (Fig 2). The reported prevalence of the OEV is highly variable, ranging from 0.46% to 58.33%.3–12 The OEV, however, has received little attention in the anatomic, surgical, and radiologic literature. Enlarged OEVs have been described in craniosynostosis, increased intracranial pressure, and transverse sinus or sigmoid sinus thrombosis.13–15
Fig 1.
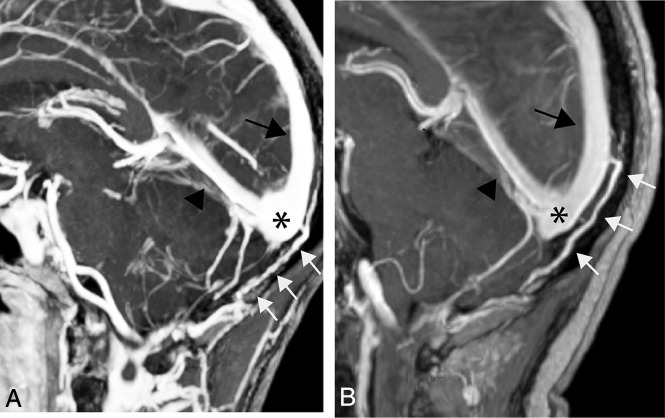
MR imaging of the craniocervical region in healthy subjects: gadolinium-enhanced 3D-T1 MPRAGE sagittal acquisitions, thick MIP reconstructions (FOV = 232 × 256 mm2, matrix = 232 × 256, slice thickness = 1 mm, slices per slab = 160–208, TR/TE = 2200/2.09–4.68 ms, bandwidth = 140–240 Hz/pixel.) Parallel acquisition was performed in the generalized autocalibrating partially parallel acquisition mode, with reference line phase encoding. A, Classic OEV origin from the torcular herophili (asterisk). The OEV courses intraosseously within the occipital squama (arrows) and exists the skull proximal to the foramen magnum to join the suboccipital veins. B, An OEV originating from the distal superior sagittal sinus. The black arrow indicates the superior sagittal sinus; the black arrowhead, the straight sinus.
Fig 2.
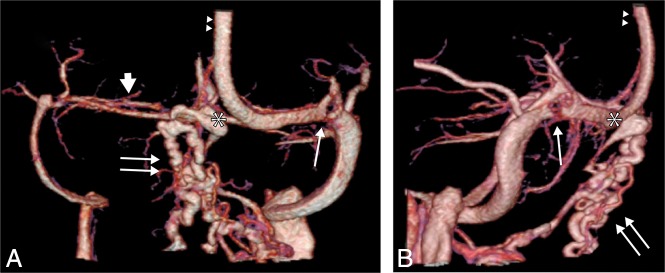
Venous phase of an SCTV obtained with a 320–detector row MDCT in a patient with pseudotumor cerebri syndrome. Posterior (A) and right lateral (B) projections. A dilated OEV (double arrows) originates from the torcular herophili (asterisk), consisting of several intraosseous and extracranial veins with various exit zones from the occipital squama. A hypoplastic transverse sinus is documented on the right side (thick arrow), and a severe transverse sinus intrinsic stenosis from an arachnoid granulation is seen on the left side (thin arrow). The double arrowheads indicate the superior sagittal sinus.
Pseudotumor cerebri syndrome (PTCS), also known as idiopathic or benign intracranial hypertension, is characterized by increased intracranial pressure, which may lead to complete loss of vision and disabilities due to intractable headaches or pulsatile tinnitus.16–20
Dural sinus venous outflow obstruction may be observed in up to 93% of patients with PTCS and plays an important role in the syndrome pathophysiology.21,22 Transverse sinus stenosis is the most sensitive sign of PTCS on MR imaging.23 Stenosis may be primary (intrinsic), principally due to thrombosis or arachnoid granulation with or without brain herniation,24 or secondary (extrinsic), mainly in relation to increased CSF pressure.25 PTCS may also be observed in dural arteriovenous fistulas due to arteriovenous shunting and venous restrictive disease.26 The cranial venous system should therefore be studied in detail in all patients with PTCS to rule out an intrinsic/extrinsic stenosis or an arteriovenous shunt and as a preplanning technique when considering dural venous sinus stent placement. Dural sinus stent placement is being used increasingly to treat PTCS that is refractory to medical management as an alternative to ventricular shunting, and has also been used in patients with PTCS with severe pulsatile tinnitus or malignant PTCS with rapidly evolving vision loss.27,28
PTCS imaging findings include an enlarged “empty sella,”29 optic nerve abnormalities (papilledema and tortuous optic nerves), dilated subarachnoid spaces around cranial nerves (optic nerve sheaths, the Meckel cave, an oculomotor nerve within the lateral wall of the longitudinal sternal stabilization system, Dorello canal, and so forth),29–31 and venous outflow restriction. Prominent OEVs may be commonly observed during clinical routine in patients with PTCS with transverse sinus stenosis and not in healthy individuals. The OEVs in patients with PTCS and transverse sinus stenosis may be increased in size because they serve as a venous collateral. The purpose of our study was to evaluate the prevalence and size of the OEV in patients with PTCS and in healthy controls and to determine whether the OEV could represent a marker of venous PTCS.
Materials and Methods
This was a retrospective case-control study. Informed consent for imaging and dural venous sinus stent placement was obtained from all patients with PTCS. In addition, all patients had given consent to being included in an institutional review board–approved data base at Johns Hopkins Hospital, authorizing analysis of data obtained during routine diagnostic and interventional clinical activity.
The cranial venous system of 46 adult patients with PTCS (group 1) was studied on subtracted CT venography (SCTV) images obtained on a 320–detector row multidetector row CT (MDCT) scanner (Aquilion ONE CT scanner; Toshiba Medical Systems, Tokyo, Japan). All patients satisfied the modified Dandy criteria for PTCS, were not pregnant, and were not on medications or had medical conditions associated with intracranial hypertension. None of these patients had undergone shunt or bariatric surgery. Technical parameters were as follow: 0.5-mm detector width, 0.25-mm reconstruction interval, 512 × 512 matrix, 160-mm FOV, 0.75-second scan rotation time, 80-kV tube voltage, and 150- to 280-mA tube current. A native head CT volume scan was obtained and used as a mask for subtraction. Thereafter, 50 mL of nonionic contrast material (iopamidol, Isovue 370; Bracco, Princeton, New Jersey) was injected intravenously at a flow rate of 6 mL/s followed by a saline flush. The dynamic volume CT angiography followed with 5 volume scans in the arterial phase at 15–25 seconds and 7 volume scans in the venous phase at 30–45 seconds postinjection. Poststenting follow-up imaging was acquired 6 months after the procedure.
A group of 92 adult patients who underwent MR imaging for routine medical studies (1.5T, Aera; Siemens Erlangen, Germany) with gadolinium-enhanced 3D-T1 MPRAGE sequences was used as controls (group 2). Inclusion criteria were adults between 18 and 60 years of age and the availability of gadolinium-enhanced 3D-T1 MPRAGE images. Excluded were patients with clinical and/or radiologic evidence of PTCS, intracranial venous thrombosis, dural sinus stenosis, intracranial arteriovenous shunts, posterior fossa surgery, posterior fossa tumor and evidence of increased intracranial pressure. MR imaging parameters were as follows: 232 × 256 mm FOV, 232 × 256 matrix, 1-mm slice thickness, 160–208 slices per slab, TR/TE = 2200/2.09–4.68 ms, 140- to 240-Hz/pixel bandwidth. A parallel acquisition was conducted in the generalized autocalibrating partially parallel acquisition mode with reference line phase encoding; 0.1 mmol/kg of gadolinium (gadoterate meglumine, Dotarem; Guerbet, Aulnay-sous-Bois, France; or gadobenate dimeglumine, MultiHance; Bracco Diagnostics, Princeton, New Jersey) was injected in each patient. When available, CT images (64–detector row MDCT, Optima MR450w with GEM Suite; GE Healthcare, Milwaukee, Wisconsin) in patients from group 2 were studied (bone algorithm reconstructions).
Image Analysis
Consensual analysis of the imaging data was performed by 2 senior radiology residents (A.H., A.P.) and a senior, board-certified neuroradiologist (D.S.M.). SCTV for group 1 and gadolinium 3D-T1 MPRAGE MR imaging of group 2 were evaluated for the presence of an OEV. When an OEV was visible, maximum diameters were measured at its proximal osseous segment in the occipital squama and extracranially immediately in front of the OEV foramen. For the control group, the presence of the osseous canal corresponding to the OEV was also assessed on CT (64–detector row MDCT) when available.
For patients who underwent transverse sinus stent placement and for whom a follow-up SCTV was available, the size of the OEV was measured in the same location before and after the procedure. Follow-up SCTV was performed within 2 days poststenting.
Statistical Analysis
The number of patients was restricted to those available in our center (monocentric study). To increase the statistical power, we recruited 2 controls for each patient. The 2:1 ratio would also compensate for the smaller frequency of visible veins in the control group to improve the statistical power of the comparison of vein sizes between groups. No pairing was performed because it would have reduced the number of subjects and would prevent any comparison on pairing variables. With 46 patients and 92 controls, a 2-sided type I error rate set at 0.05, an expected proportion of controls with a visible vein equal to 30%, and an expected proportion of patients with a visible vein equal to 65%, the statistical power would be to 45%.
Data are presented as median and range for continuous variables and as a percentage for frequency data. Statistical analysis of frequency data was performed using a Fisher exact test. Continuous variables were compared using a Student t test. A P value ≤ .05 was considered statistically significant. Statistical analysis was performed using the Statistical Toolbox in Matlab (MathWorks, Natick, Massachusetts).
Results
Patient demographics are summarized in Table 1. Patients with PTCS and control subjects were similar in overall profile. Patients with PTCS had a slightly greater preponderance of women.
Table 1:
Characteristics of patients with PTCS and the control group
| PTCS (n = 46) | Control (n = 92) | P Value | |
|---|---|---|---|
| Age (mean) (yr) | 35.6 ± 9.9 | 41.1 ± 12.3 | .01 |
| Female/male | 40:6 | 62:30 | .01 |
| CSF opening pressure (mean) (range) (cm H2O) | 34 ± 9 (22–65) | ||
| (n = 44/46) | |||
| Patients treated with stenting | 29/46 | ||
| Prestenting pressure gradient (mean) (range) (mm Hg) | 9 (6–15) | ||
| (n = 29/46) | |||
| Poststenting pressure gradient (No.) (range) (mm Hg) | 1 (0–2) | ||
| (n = 29/46) |
Forty-six patients were included in the PTCS group (87% were women with an average age of 36 years), and MR imaging studies from 92 consecutive patients meeting the selection criteria were included in the control group (67% were women with an average age of 41 years). No patient was further excluded from analysis after inclusion.
An OEV was observed in 30 of the 46 patients in group 1 (65.2%) and 29 of the 92 patients in group 2 (31.5%) (P < .0003). The prevalence of an OEV for men and women was similar in groups 1 and 2.
The average diameter of the OEV in its osseous segment (proximal) was 2.3 mm (range, 1–7 mm) in group 1 and 1.6 mm (range, 0.5–3 mm) in group 2 (P < .0049). Extracranially, at the exit of the OEV foramen, the average OEV diameter was 3.3 mm in group 1 (range, 1.8–10 mm) and 2.3 mm (range, 1–7 mm) in group 2 (P < .0001). The average proximal and distal OEV maximum diameters were therefore both significantly larger in group 1. OEV characteristics are summarized in Table 2.
Table 2:
Comparison of OEV characteristics of patients with PTCS and the control group
| Group 1 (PTCS) (n = 46) | Group 2 (Control) (n = 92) | P Value | |
|---|---|---|---|
| Prevalence of OEV (No.) | 65.2% (30) | 31.5% (29) | <.0003 |
| Male | 4/6 (66.6%) | 9/30 (30.0%) | |
| Female | 24/40 (60.0%) | 20/62 (32.2%) | |
| Osseous segment diameter (mean) (range) (mm) | 2.3 (1–7) | 1.6 (0.5–3) | <.0049 |
| Extracranial segment diameter (mean) (range) (mm) | 3.3 (1.8–10) | 2.3 (1–7) | <.0001 |
In group one, 29 of the 46 patients (63%) underwent transverse sinus stent placement; 17 had a poststenting control CT venography at the time of review (Fig 3). Poststent placement reduction in the size of the OEV was observed in 9/17 cases (53%). In 2 cases, the reduction was only visible in the extracranial portion, and in 1 case, the OEV was no longer visible. The average maximum diameters of the OEV before and after stent placement were, respectively, 2.6 versus 1.8 mm proximally (intraosseous segment, P < .06) and 3.7 versus 2.6 mm distally (extracranial portion, P < .0045). OEV characteristics results before and after stent placement are summarized in Table 3.
Fig 3.
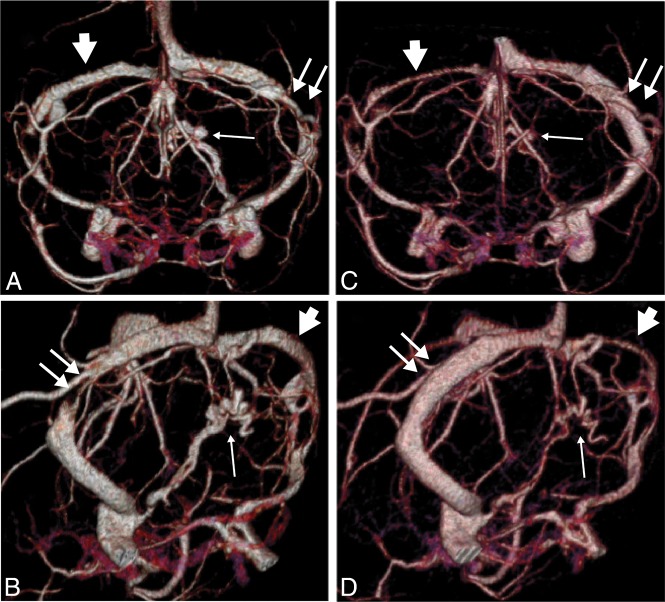
Venous phase of an SCTV with a 320–detector row MDCT in a patient with pseudotumor cerebri syndrome before (A and B) and after (C and D) left transverse sinus stent placement, anterior Towne projections (A and C), and left posterior oblique projections (B and D). There is a marked reduction of the OEV (small arrow) after stent placement in the left transverse sinus (double arrows), which has recovered its normal caliber. Note the size reduction of the right transverse sinus (arrowhead) proximal to an intrinsic stenosis after stent placement due to preferential drainage of the superior sagittal sinus into the stented transverse sinus.
Table 3:
Comparison of OEV characteristics of patients with PTCS before and after transverse sinus stenting
| Before Stenting (n = 17) | After Stenting (n = 17) | P Value | |
|---|---|---|---|
| Osseous segment diameter (mean) (range) (mm) | 2.6 (1.5–4) | 1.8 (1–2.1) | <.06 |
| Extracranial segment diameter (mean) (range) (mm) | 3.7 (2–7) | 2.6 (1–3.25) | <.0045 |
The exit point of the OEV was, in both groups, always located between the external occipital protuberance and the foramen magnum. Multiple OEVs were found in 3.2% of cases in group 2, two OEVs on 2 occasions and a triple OEV once. On 1 occasion, the OEV took its origin from the distal superior sagittal sinus and not the torcular herophili (Fig 1B).
At the time of data collection for group 1, a sufficient number of SCTVs of healthy patients that could be used as a healthy control population for group 2 were not available. Thus, group 2 was based on MR imaging data using gadolinium-enhanced 3D-T1 MPRAGE. Gadolinium-enhanced 3D-T1 MPRAGE provides very good depiction of diploic veins and was thought to reliably demonstrate the prevalence of an OEV.32 In group two, 75% of patients had CT data available. One hundred percent of OEVs observed on MR imaging were visible on CT. In 4 patients (4.3%), an OEV canal was visible on CT but no visible OEV was found on MR imaging, which could be due to the OEV having become atretic with time. MR imaging could therefore be less sensitive than CT for detecting OEVs in a small percentage of patients, though in these cases, the OEV may not be functional (atretic).
Discussion
Venous outflow obstruction is increasingly recognized as an etiology of PTCS, and transverse sinus stenosis is considered a sensitive sign of the condition.23 The observation of very prominent OEVs in patients with PTCS led us to investigate the size and prevalence of OEVs in these patients and in healthy controls.
The OEV has received little attention in the literature. The few anatomic studies available were conducted on dry skulls. OEV foramina prevalence varied significantly among studies, ranging from as low as 0.46% up to 58% (On-line Table). In our study, an OEV was present in 65% of patients with PTCS and 30% of control subjects. In terms of OEV prevalence, 1 study presented results similar to those in our PTCS group (group 1),11 and another showed results similar to those in our control group (group 2).12 Overall, a higher OEV prevalence was found in both groups compared with the prevalence previously reported in the anatomic literature. This prevalence is surprising considering that even the smallest bone foramina should be detectable on dry skulls. On the other hand, occipital squama foramina may account not only for OEVs but also exit zones of regional diploic veins, potentially leading to OEV prevalence overestimation on dry skull specimens.
When we considered discrepancies in size measurements obtained on CT and MR imaging,33 OEVs were significantly larger in patients with PTCS (group 1), all of whom had transverse sinus stenosis, than in control subjects without venous outflow obstruction (group 2). Surprisingly, however, the prevalence of OEV in patients with PTCS was significantly higher than in control subjects (65.2% versus 31.5%). OEVs missed by the imaging protocol in group 2 consisting of gadolinium 3D-T1 MPRAGE and dry CT (in most cases) could explain this discrepancy, though both techniques successfully detected OEVs as small as 0.5 mm. Alternatively, venous hypertension (demonstrated by intravenous pressure measurements in all patients with PTCS) may recanalize atretic OEVs or render more conspicuous very small OEVs that would otherwise be undetectable in normal conditions.
The OEV is the only potential venous collateral pathway of the posterior fossa in cases of transverse sinus or proximal sigmoid sinus stenosis aside from the parietal emissary veins of Santorini, which were not studied here. Other posterior fossa emissary veins such as the mastoid or posterior condylar emissary veins1 are located downstream from the transverse sinus stenosis generally encountered in PTCS. Although the size of OEVs significantly increases in PTCS, it did not represent a functionally efficient derivation pathway because all our patients were clinically symptomatic. The ability of the OEV to represent a successful collateral pathway is likely limited by its intraosseous portion, which probably restricts the degree of OEV dilation. Indeed, OEV diameters were, in all cases, larger extracranially than in the osseous portion. The role of the OEV as a collateral venous pathway is, however, supported by its regression in 53% of the stented patients with PTCS.
The OEV anatomy in group 2 was otherwise consistent with classic descriptions. Its origin was from the distal superior sagittal sinus on 1 occasion (1%), and multiple OEVs were observed in 3 healthy subjects (2 double OEVs, 1 triple OEV).
There are limitations to this study. First, the retrospective nature of the analysis and lack of blinding could limit the generalization of our findings. Different imaging modalities were used to compare a group of patients with PTCS (SCTV) with a control group of healthy individuals (MR imaging). The study design sizes of OEVs obtained through CT and MR imaging measurements could, therefore, be partly explained by differences in size rendering between the 2 techniques, especially given the small size of the evaluated anatomic structures (several millimeters). The sensitivity of MR imaging in detecting the OEV was also likely lower than in CT (4.3% of OEV canals on CT did not contain an OEV on MR imaging), possibly due to atretic OEVs. This likelihood could explain the higher prevalence of OEVs in the PTCS group than in the control group. Indeed, elevated venous pressure in dural sinuses proximal to the transverse sinus stenosis could lead to recanalization of an atretic OEV.
Conclusions
The OEV diameter in patients with PTCS is larger than in healthy subjects, a finding consistent with its role as a collateral venous pathway in transverse sinus stenosis. An OEV is more frequent in patients with PTCS than in healthy subjects, even though the prevalence of OEVs in the latter group was found to be higher than previously reported in the literature. A prominent OEV on CT should prompt the radiologist to look for other signs of PTCS and suggest a CT- or MR imaging–based venous study. Prominent occipital emissary veins may be a valuable secondary sign prompting further investigation.
ABBREVIATIONS:
- OEV
occipital emissary vein
- MDCT
multidetector row CT
- PTCS
pseudotumor cerebri syndrome
- SCTV
subtracted CT venography
Footnotes
Disclosures: Abhay Moghekar—UNRELATED: Consultancy: Orbees Inc, Putnam Associates, Comments: Market research about hydrocephalus, market research about stroke; Expert testimony: Koskoff and Beider DeLuca & Weizenbaum Ltd, Comments: Medicolegal consultancy; Payment for development of educational presentations: NEJM-Knowledge Plus, Comments: Developed neurology online questions; Travel/accommodations/meeting expenses unrelated to activities listed: Spinal CSF Leak Foundation, Comments: Travel and accommodation reimbursed for guest lecture. Philippe Gailloud—UNRELATED: Consultancy: Cerenovus; Grants/grants pending: Siemens*; Patents (planned, pending or issued): Artventive; Stock/stock options: Artventive. *Money paid to institution.
REFERENCES
- 1. San Millán Ruíz D, Gailloud P, Rüfenacht DA, et al. . The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 2002;23:1500–08 [PMC free article] [PubMed] [Google Scholar]
- 2. Pekçevik Y, Pekçevik R. Why should we report posterior fossa emissary veins? Diagn Interv Radiol 2014;20:78–81 10.5152/dir.2013.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd GI. The emissary foramina of the cranium in man and the anthropoids. J Anat 1930;65:108–21 [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma PK, Malhotra VK, Tewari SP. Emissary occipital foramen. Anat Anz 1986;162:297–98 [PubMed] [Google Scholar]
- 5. Premsagar IC, Lakhtakia PK, Bisaria KK. Occipital emissary foramen in Indian skulls. J Anat 1990;173:187–88 [PMC free article] [PubMed] [Google Scholar]
- 6. Gözil R, Kadioglu D, Calgüner E. Occipital emissary foramen in skulls from central Anatolia. Acta Anat (Basel) 1995;153:325–26 10.1159/000147742 [DOI] [PubMed] [Google Scholar]
- 7. Louis RG Jr, Loukas M, Wartmann CT, et al. . Clinical anatomy of the mastoid and occipital emissary veins in a large series. Surg Radiol Anat 2009;31:139–44 10.1007/s00276-008-0423-5 [DOI] [PubMed] [Google Scholar]
- 8. Akram Hossain SM, Lutfor Rahman MK. Occipital emissary foramen in Bangladeshi skulls. Pak J Med Sci 2001;17:156–58 [Google Scholar]
- 9. Murlimanju BV, Prabhu LV, Pai MM, et al. . Occipital emissary foramina in human skulls: an anatomical investigation with reference to surgical anatomy of emissary veins. Turk Neurosurg 2011;21:36–38 [PubMed] [Google Scholar]
- 10. Singhal S, Ravindranath R. Occipital emissary foramina in South Indian modern human skulls. ISRN Anat 2013;2013:727489 10.5402/2013/727489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Subashri A, Thenmozhi MS. Occipital emissary foramina in human adult skull and their clinical implications. Research Journal of Pharmacy and Technology 2016;9:716 10.5958/0974-360X.2016.00135.9 [DOI] [Google Scholar]
- 12. Kabilamurthi S. Occipital emissary foramen? A morphometric analysis. Int J Sci Res 2017;6:1853–54 [Google Scholar]
- 13. Hayward R. Venous hypertension and craniosynostosis. Childs Nerv Syst 2005;21:880–88 10.1007/s00381-004-1114-0 [DOI] [PubMed] [Google Scholar]
- 14. Chynn KY. Occipital emissary vein enlargement: a sign of increased intracranial pressure. Radiology 1963;81:242–47 10.1148/81.2.242 [DOI] [PubMed] [Google Scholar]
- 15. Salem M, Dolati P, Fusco MR, et al. . Abnormal large central occipital emissary vein: a case report and review of literature. Cureus 2016;8:e60 10.7759/cureus.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri: population studies in Iowa and Louisiana. Arch Neurol 1988;45:875–77 10.1001/archneur.1988.00520320065016 [DOI] [PubMed] [Google Scholar]
- 17. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain 1991;114(Pt 1A):155–80 [PubMed] [Google Scholar]
- 18. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59:1492–95 10.1212/01.WNL.0000029570.69134.1B [DOI] [PubMed] [Google Scholar]
- 19. Lansley JA, Tucker W, Eriksen MR, et al. . Sigmoid sinus diverticulum, dehiscence, and venous sinus stenosis: potential causes of pulsatile tinnitus in patients with idiopathic intracranial hypertension? AJNR Am J Neuroradiol 2017;38:1783–88 10.3174/ajnr.A5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kastanioudakis I, Konitsiotis S, Asproudis I, et al. . Venous pulsatile tinnitus due to pseudotumor cerebri syndrome in a young morbid obese female. Hippokratia 2013;17:383 [PMC free article] [PubMed] [Google Scholar]
- 21. Kumpe DA, Bennett JL, Seinfeld J, et al. . Dural sinus stent placement for idiopathic intracranial hypertension. J Neurosurg 2012;116:538–48 10.3171/2011.10.JNS101410 [DOI] [PubMed] [Google Scholar]
- 22. Farb RI, Vanek I, Scott JN, et al. . Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003;60:1418–24 10.1212/01.WNL.0000066683.34093.E2 [DOI] [PubMed] [Google Scholar]
- 23. Morris PP, Black DF, Port J, et al. . Transverse sinus stenosis is the most sensitive MR imaging correlate of idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2017;38:471–77 10.3174/ajnr.A5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malekzadehlashkariani S, Wanke I, Rüfenacht DA, et al. . Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology 2016;58:443–57 10.1007/s00234-016-1662-5 [DOI] [PubMed] [Google Scholar]
- 25. Morris PP, Lachman N, Black DF, et al. . Increased curvature of the tentorium cerebelli in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2017;38:1789–93 10.3174/ajnr.A5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cognard C, Casasco A, Toevi M, et al. . Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry 1998;65:308–16 10.1136/jnnp.65.3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmed RM, Wilkinson M, Parker GD, et al. . Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol 2011;32:1408–14 10.3174/ajnr.A2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elder BD, Goodwin CR, Kosztowski TA, et al. . Venous sinus stenting is a valuable treatment for fulminant idiopathic intracranial hypertension. J Clin Neurosci 2015;22:685–89 10.1016/j.jocn.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 29. Bialer OY, Rueda MP, Bruce BB, et al. . Meningoceles in idiopathic intracranial hypertension. AJR Am J Roentgenol 2014;202:608–13 10.2214/AJR.13.10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. San Millán D, Kohler R. Enlarged CSF spaces in pseudotumor cerebri. AJR Am J Roentgenol 2014;203:W457–58 10.2214/AJR.14.12787 [DOI] [PubMed] [Google Scholar]
- 31. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol 2011;32:1986–93 10.3174/ajnr.A2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jivraj K, Bhargava R, Aronyk K, et al. . Diploic venous anatomy studied in-vivo by MRI. Clin Anat 2009;22:296–301 10.1002/ca.20767 [DOI] [PubMed] [Google Scholar]
- 33. Khandelwal N, Agarwal A, Kochhar R, et al. . Comparison of CT venography with MR venography in cerebral sinovenous thrombosis. AJR Am J Roentgenol 2006;187:1637–43 10.2214/AJR.05.1249 [DOI] [PubMed] [Google Scholar]