Abstract
BACKGROUND AND PURPOSE:
The significance of renal contrast on CT myelography is uncertain. This project examined different patient populations undergoing CT myelography for the presence of renal contrast to determine whether this finding is of diagnostic value in spontaneous intracranial hypotension.
MATERIALS AND METHODS:
Four groups of patients were analyzed for renal contrast on CT myelography. The control group underwent CT myelography for reasons other than spontaneous intracranial hypotension (n = 47). Patients in study group 1 had spontaneous intracranial hypotension but CT myelography negative for dural CSF leak and CSF venous fistula (n = 83). Patients in study group 2 had spontaneous intracranial hypotension and CT myelography positive for dural CSF leak (n = 44). Patients in study group 3 had spontaneous intracranial hypotension and CT myelography suggestive of CSF venous fistula due to a hyperdense paraspinal vein (n = 17, eleven surgically confirmed).
RESULTS:
Renal contrast was present on the initial CT myelography in 0/47 patients in the control group, 10/83 patients in group one, 1/44 patients in group 2, and 7/17 patients in group 3. Renal contrast on initial CT myelography in patients with suspected or surgically confirmed CSF venous fistula was significantly more likely than in patients with a dural CSF leak (P = .0003).
CONCLUSIONS:
Renal contrast on initial CT myelography was seen only in patients with spontaneous intracranial hypotension. This was more common in confirmed/suspected CSF venous fistulas compared with dural leaks. Early renal contrast in patients with spontaneous intracranial hypotension should prompt scrutiny for a hyperdense paraspinal vein, and, if none is found, potentially advanced diagnostic studies.
Spontaneous intracranial hypotension (SIH) typically presents clinically with an orthostatic headache but can also have a plethora of additional associated symptoms such as nausea, visual difficulties, tinnitus, and, with time, development of gait disturbance, personality change, and decreased level of consciousness.1 In most cases, SIH is caused by a spinal dural CSF leak. In some patients, the site of the CSF leak cannot be identified by current imaging techniques, including standard and dynamic CT myelography (CTM) and off-label intrathecal gadolinium MR myelography.
Recently, CSF venous fistula (CVF) has been described as a cause of SIH, in which there is a direct connection between the CSF and a draining vein.2 Schievink et al2 described 3 patients who had a direct fistula between the subarachnoid space and spinal epidural veins on digital subtraction myelography. Subsequently, a hyperdense paraspinal vein sign on CTM has been described as a similar indicator of a CVF.3 In this study, the 3 patients with the hyperdense paraspinal vein sign had surgically confirmed CVF. The prevalence of this standard CTM marker of CVF has subsequently been evaluated, occurring in 7% of patients with SIH who do not have evidence of a dural CSF leak.4
In nuclear medicine indium-111 (111In) DTPA cisternography, early renal collecting system activity before 4 hours after injection is considered a sign of CSF leak.5 We have noticed the presence of renal collecting system contrast (referred to as “renal contrast” from this point forward) on CTM performed for SIH and have wondered whether it is of any clinical significance because it is presumably a CTM correlate to early radiotracer activity in the renal collecting system. We have questioned whether early renal contrast on CTM could be a secondary sign of CSF leak and/or CVF. The purpose of this project was to investigate different patient populations undergoing CTM for the presence of renal contrast to determine whether this finding is of diagnostic value in SIH.
Materials and Methods
Institutional review board approval with waived consent was obtained for this Health Insurance Portability and Accountability Act–compliant retrospective research study. All included patients provided authorization for data to be used in research.
Patient Selection
Four groups of patients were identified, including a control group and 3 study groups: study group 1, study group 2, and study group 3.
The control group included patients who underwent CTM for reasons other than SIH. Patients in the control group were derived from a data base search of our single institution electronic medical record to identify approximately 50 patients who underwent CTM for a non-SIH indication in 2015.
Study group 1 included patients with SIH and CTM negative for a dural CSF leak or the hyperdense paraspinal vein sign. Patients in study group 1 were derived from a data base search to identify approximately 100 patients who underwent CTM for an indication of SIH from 2011 to 2015 without evidence of a dural CSF leak or the hyperdense paraspinal vein sign.
Study group 2 included patients with SIH and CTM positive for a dural CSF leak. Patients in study group 2 were derived from a data base search to identify approximately 50 consecutive patients with SIH and CTM positive for dural CSF leak from 2011 to 2014.
Study group 3 included patients with SIH and CTM suggestive of CVF due to the presence of a hyperdense paraspinal vein. Patients in study group 3 were derived in 2 ways: Some patients were found via an electronic medical record data base search for patients with SIH and CTM positive for a hyperdense paraspinal vein. The remaining patients in study group 3 were obtained from a prior retrospective review of data on CTMs performed for SIH at our institution, in which the hyperdense paraspinal vein sign was present convincingly but had been found retrospectively because at the time of their CTM, CVF was not a recognized entity. All patients in study group 3 were further evaluated by clinical chart review to note whether CVF had been surgically confirmed following CTM, and a subgroup analysis was performed.
Patients referred to CTM for SIH had been diagnosed clinically by neurologists at our institution who frequently see patients for the indications of SIH. Patients who received IV contrast before CTM the day of or day before the procedure were excluded. Note was made of whether the renal collecting systems were included in the field of imaging, and patients in whom the renal collecting systems could not be at least partially visualized were also excluded. Demographic information of the patients, including age and sex, was recorded. Type and volume of intrathecal contrast injected for each CTM were noted.
Imaging Review
Each CTM was retrospectively analyzed for renal contrast by a board-certified neuroradiologist from our institution, including initial CTM images and delayed images (if obtained). Although the renal contrast finding is subjective, reviewers were asked to only grade the CTMs as positive for this finding if they had the highest level of suspicion (ie, if they were convinced and would interpret the studies as positive for renal contrast clinically). The reviewing radiologists were blinded to the indication for the CTM and the CTM group assignment. Any case deemed positive for renal contrast was confirmed by a second neuroradiologist. If renal contrast was present on the initial CTM, the initial CTM was reviewed for contrast leakage at the lumbar puncture site because this has been suggested as a potential cause of false-positive early renal collecting system radiotracer activity on nuclear medicine 111In DTPA cisternography.6
The presence of a hyperdense paraspinal vein was evaluated subjectively. In cases of a hyperdense paraspinal vein identified in prior retrospective review of CTMs initially interpreted as negative,4 reviewers had been asked to look for a linear/curvilinear opacified structure extending from the thecal sac or a nerve root sleeve suggesting a hyperdense paraspinal vein.3 Only cases with a high index of suspicion were graded as positive (ie, if the case would be or had been interpreted positive clinically), and any case deemed positive for a hyperdense paraspinal vein was confirmed by 2 additional neuroradiologists. In cases of a hyperdense paraspinal vein that was identified by an electronic medical record data base search (ie, cases that had prospectively been called clinically), a second neuroradiologist reviewed the case to confirm the finding.
Times from injection of myelographic contrast to initial and delayed (if performed) CTM were recorded. The patient groups were compared for rates of the presence of renal contrast, including on both initial and delayed CTM images. In cases positive for renal contrast on the initial CTM with concurrent MR myelography performed, the MR myelogram was also evaluated for the presence of renal contrast.
Statistical Analysis
Comparison between rates of the presence of renal contrast on initial and delayed CTM among the groups was performed using a 2-tailed Fisher exact test. Comparison of the time between intrathecal contrast injection and initial CTM imaging was performed using a 2-tailed Mann-Whitney U test. A P value < .05 was deemed statistically significant.
Results
A summary of the results from the control group and the 3 study groups is provided in the Table. After we excluded patients with IV contrast before CTM (n = 19) and patients in whom the renal collecting systems could not be at least partially visualized (n = 1), the total study population was 191 patients.
Summary of results from the control and 3 study groups
| Group | Control Group: Non-SIH | Study Group 1: SIH+/CSF Leak−/ Hyperdense Vein− | Study Group 2: SIH+/CSF Leak+ | Study Group 3: SIH+/ Hyperdense Vein+ |
|---|---|---|---|---|
| Included patients (No.) | 47 | 83 | 44 | 17 |
| Mean age (range) (yr) | 66 (35–91) | 59 (31–87) | 54 (28–71) | 56 (37–84) |
| Male sex | 62% | 45% | 36% | 41% |
| Mean intrathecal contrast volume injected (range) (mL) | 14 (10–20) | 15 (12–20) | 13 (6–20) | 14 (12–18) |
| Initial CTM | ||||
| All patients: mean time from contrast injection to initial CTM (range) (min) | 35 (19–101) | 40 (2–113) | 20 (2–87) | 41 (4–83) |
| Patients with renal contrast (No.) (%) | 0/47 (0%) | 10/83 (12%) | 1/44 (2%) | 7/17 (41%) |
| Patients with renal contrast: mean time from contrast injection to initial CTM (range) (min) | NA | 61 (17–96) | Contrast injection time not documented in this patient | 37 (4–62) |
| Delayed imaging | ||||
| Patients with delayed imaging (No.) (%) | 0/47 (0%) | 73/83 (88%) | 11/44 (25%) | 15/17 (88%) |
| All patients: mean time from contrast injection to delayed CTM (range) (min) | NA | 199 (79–364) | 162 (93–325) | 195 (100–386) |
| Patients with renal contrast (No.) (%) | NA | 23/73 (32%) | 3/11 (27%) | 8/15 (53%) |
| Patients with renal contrast: mean time from contrast injection to delayed imaging (range) (min) | NA | 220 (126–364) | 211 (97–325) | 184 (100–386) |
Note:—NA indicates not applicable.
Control Group (Non-SIH)
Forty-eight patients were identified. One (2%) was excluded due to IV contrast before CTM, leaving 47 patients in the control group. All patients received iohexol, either Omnipaque 180 (60%) or Omnipaque 240 (40%) iodinated contrast (GE Healthcare, Piscataway, New Jersey) intrathecally. Of the 47 patients, none (0%) had renal contrast on the initial CTM. No patients had delayed imaging performed, because the non-SIH indication did not necessitate it.
Study Group 1 (SIH, CTM Negative for Dural CSF Leak or Hyperdense Paraspinal Vein)
Ninety-four patients were identified in group 1. Eleven (12%) were excluded due to IV contrast before CTM, leaving 83 patients in study group 1. All patients received either Omnipaque 180 (35%) or Omnipaque 240 (65%). Of the 83 patients, 10 (12%) had renal contrast on the initial CTM (4 of whom had contrast leakage at the injection site) at a mean time of 61 minutes postinjection (range, 17–96 minutes). Seventy-three (88%) of these 83 patients had delayed imaging, with 23 (32%) of 73 having renal contrast at a mean time of 220 minutes postinjection (range, 126–364 minutes).
Study Group 2 (SIH, CTM Positive for Dural CSF Leak)
Fifty-one patients were identified in group 2. Seven (14%) were excluded, 6 (12%) due to IV contrast before CTM, and 1 (2%) due to only thoracic imaging being performed without inclusion of the kidneys, leaving 44 patients in study group 2. All patients received either Omnipaque 180 (70%) or Omnipaque 240 (30%). Of the 44 patients, 1 (2%) had renal contrast on initial CTM and did not have contrast leakage at the injection site. Unfortunately, the time of intrathecal contrast injection was not documented in this patient, so the postinjection time at which renal contrast was seen on the initial CTM could not be determined. Only 11 (25%) of these 44 patients had delayed imaging because a dural CSF leak was usually identified on the initial CTM, with 3 (27%) of 11 having renal contrast at a mean time of 211 minutes postinjection (range, 97–325 minutes).
Study Group 3 (SIH, CTM Positive for Hyperdense Paraspinal Vein)
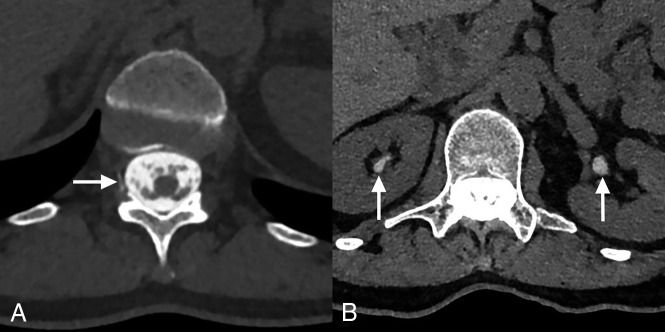
Nineteen patients were identified in group 3, twelve (63%) through data base search and 7 (37%) who had previously been identified in a retrospective review after the hyperdense paraspinal vein sign had been described. Two (11%) were excluded due to IV contrast before CTM, leaving 17 patients in study group 3. All patients received either Omnipaque 180 (29%) or Omnipaque 240 (71%). Of the 17 patients, seven (41%) had renal contrast on the initial CTM (Figure) at a mean time of 37 minutes postinjection (range, 4–62 minutes), and only 1 had contrast leakage at the injection site. Fifteen (88%) of these 17 patients had delayed imaging, with 8 (53%) of 15 having renal contrast at a mean time of 184 minutes postinjection (range, 100–386 minutes).
FIGURE.
Axial CT images from the initial CTM for a study group 3 patient with a hyperdense paraspinal vein sign (arrow, A) at T12–L1 on the right (better visualized while scrolling through consecutive images) and renal contrast (arrows, B).
Of the 17 patients in study group 3, eight (47%) received an injection of intrathecal normal saline as part of their myelogram, and 5 (50%) of these 8 patients also received 0.5 mL of gadopentetate dimeglumine, Magnevist (Bayer HealthCare Pharmaceuticals, Wayne, New Jersey), as part of their myelogram. These were injected as part of advanced myelographic techniques (MR and positive pressure myelography) looking for CVF. The mean volume of saline injected in these patients was 43 mL (range, 16–70 mL). Of the 4 patients who underwent MR myelography, renal contrast findings on MR myelography were concordant with those on CT myelography when both were available at similar time points. One patient who underwent MR myelography had renal contrast on the initial CTM, which was also present on initial MR myelography. Of the 3 patients who underwent MR myelography without renal contrast on the initial CTM, 2 did not have early MR imaging and 1 did not have the renal collecting systems included in the MR imaging FOV. When we excluded the 8 patients who underwent positive pressure ± MR myelography from the analysis due to the potential for false-positives resulting from increased intrathecal pressure, four (44%) of the 9 remaining patients had renal contrast on the initial CTM at a mean time of 28 minutes postinjection (range, 4–44 minutes). Seven (78%) of these 9 patients had delayed imaging, with 3 (43%) of 7 having renal contrast (mean time, 129 minutes; range, 100–147 minutes).
Study Group 3: Surgically Confirmed Subgroup
Subgroup analysis of the 11 patients with surgically confirmed CVF showed that 4 (36%) of 11 had renal contrast on initial imaging at a mean time of 49 minutes postinjection (range, 34–62 minutes). Nine (82%) of these 11 patients had delayed imaging, with 5 (56%) of 9 having renal contrast at a mean time of 217 minutes postinjection (range, 150–386 minutes).
Study Group 3: Not Surgically Confirmed Subgroup
Subgroup analysis of the 6 patients with a hyperdense paraspinal vein who did not proceed to an operation showed that 3 (50%) of 6 had renal contrast on initial imaging at a mean time of 22 minutes postinjection (range, 4–40 minutes). Six (100%) of these 6 patients had delayed imaging, with 3 (50%) of 6 having renal contrast at a mean time of 129 minutes (range, 100–147 minutes).
RESULTS
Among the patients with SIH in whom CTM identified the etiology of the syndrome, renal contrast on the initial CTM with suspected or surgically confirmed CVF was significantly more likely than with a dural CSF leak (P < .001). This finding remained statistically significant even with exclusion of the 8 patients in study group 3 who received concurrent intrathecal normal saline ± Magnevist (P = .002). Renal contrast on delayed CTM with suspected or surgically confirmed CVF was seen more commonly than with a dural CSF leak, but this difference was not statistically significant (P = .25).
In the setting of suspected CVF with surgical confirmation, renal contrast on the initial CTM was significantly more likely than with a dural CSF leak (P < .004). Renal contrast on delayed CTM was also seen more commonly, but this difference was not statistically significant (P = .36).
In those suspected of CVF on CTM who did not undergo surgical confirmation, renal contrast on the initial CTM was significantly more likely than with a dural CSF leak (P < .004). Renal contrast on delayed CTM was also seen more commonly, but this difference was not statistically significant (P = .60).
Renal contrast was seen only on initial CTM in patients with SIH; it was not seen in any of the patients in the control group. This finding was statistically significant when comparing all patients with SIH with the control group (P = .008) and when comparing the hyperdense paraspinal vein group with the control group (P < .001), and this difference remained statistically significant when excluding patients with SIH in whom renal contrast was seen on the initial CTM in the setting of contrast leakage from the injection site in all patients with SIH (P = .04) and in the hyperdense paraspinal vein group (P < .001). There was also a significant difference between the SIH-positive, CTM-negative group and the control group with regard to renal contrast on initial CTM (P = .010), which was not statistically significant when excluding the patients with SIH in whom there was contrast leakage from the injection site (P = .08). There was no difference between the rates of renal contrast on the initial CTM in the control group versus the group positive for a dural leak.
There was a significant difference in the time between intrathecal contrast injection and the initial CTM imaging in the dural CSF leak group compared with each of the other 3 groups (P = .004 when comparing the dural CSF leak with hyperdense paraspinal vein groups, and P < .001 when comparing the dural CSF leak group with the control and SIH-positive/leak-negative groups). There was no significant difference between the time to initial CTM imaging when comparing the control, SIH-positive/leak-negative, and hyperdense paraspinal vein groups.
Discussion
The presence of renal contrast on the initial CTM was more likely in patients with a hyperdense paraspinal vein sign and in those with surgically confirmed CVF than in patients with a dural CSF leak. This increased prevalence was also seen on delayed imaging, but the difference was not statistically significant. None of the patients undergoing CTM for non-SIH indications (n = 47) had renal contrast on the initial CTM. Given that renal contrast on the initial CTM was seen only in patients with SIH, this finding is compatible with abnormal CSF physiology (a CSF-leak state). Furthermore, renal contrast on the initial CTM may be a predictor of CVF because it was frequently seen in these patients and was only present in 1 patient with a dural CSF leak. Intuitively, the presence of early renal contrast makes sense in the setting of CVF because myelographic contrast directly enters the venous system and would therefore be cleared sooner by the kidneys than in a dural CSF leak, in which contrast first enters the epidural space. This phenomenon seems similar to early renal uptake on nuclear medicine cisternography with a CSF leak.6,7
The physiology and dynamics of absorption of iodinated contrast from the CSF following intrathecal injection have been previously described.8 The iodinated contrast absorption rate increases with an increased CSF absorption rate and more rapid mixing of contrast material with the CSF. More rapid mixing would be expected to occur in CT myelography because contrast is run throughout the spinal canal by tilting the table. Contrast absorption begins nearly immediately following injection through the spinal arachnoid villi and granulations, though this clearly does not result in renal collecting system opacification on the initial CTM in most patients. In patients with SIH, there may be increased CSF production in an attempt to compensate for a low CSF pressure state, as well as decreased venous pressure/venous dilation as can sometimes be seen on imaging studies.9 These could potentially increase the rate of CSF absorption and therefore intrathecal contrast absorption in patients with SIH. Although this phenomenon could potentially contribute to early renal contrast in patients with SIH, it would not explain why early renal contrast is seen more commonly in CVF than in a dural CSF leak, and this seems more likely due to contrast directly entering a vein rather than the epidural space in a CVF.
In our study, 12% of patients with SIH but a CTM negative for leak (standard dural type or CVF) had renal contrast on the initial CTM. This may represent a patient population with undetected CVF. Given that CVF is a relatively new diagnosis, potentially many cases of CVF are currently not being recognized, particularly on conventional CTM. For example, a series of patients studied by Schievink et al10 showed that CVF was identified on digital subtraction myelography in 10 (19%) of 53 patients without evidence of CSF leak on CTM. Prior studies have shown that CVF can respond successfully to surgical treatment, thus making the diagnosis of the utmost importance because many of these patients have debilitating symptoms.2,3,10 Perhaps the 12% of patients in our study with SIH with CTM negative for leak but renal contrast present on initial CTM would be those most likely to benefit from more advanced myelographic techniques to search for CVF, particularly if their condition is debilitating. Such techniques include digital subtraction myelography and intrathecal gadolinium myelography, potentially using positive pressure for either technique.
The presence of renal contrast and a hyperdense paraspinal vein was subjectively evaluated, though only graded positive if there was a high index of suspicion. To our knowledge, there is no standard threshold Hounsfield unit value in the literature indicating that the renal collecting system are positive for renal contrast, hence the subjective evaluation. While possible Hounsfield unit threshold values have been suggested for identifying the presence of a hyperdense paraspinal vein,11 in our practice, we have found that the presence of a hyperdense paraspinal vein sign on CTM can be quite subtle, sometimes only seen using dual-energy CT, thus limiting the practical utility of a specific Hounsfield unit threshold. Additionally, some CVFs are better visualized on MR myelography in cases of concurrently performed CTM and MR myelography as was also the case in our study.
Limitations of this study include the limited patient sample size, with most notably only 17 patients with a hyperdense paraspinal vein sign and only 11 of these proceeding to an operation with confirmatory surgical findings of CVF. The control group was also relatively small; this feature could potentially result in rare cases of missed renal contrast on initial CTM in patients without SIH, though the statistical analysis does demonstrate a significant difference in renal contrast on the initial CTM between all patients with SIH and the control group, and an even more significant difference between the hyperdense paraspinal vein group and the control group. While a few patients with SIH with an initial CTM positive for renal contrast had contrast leakage from the lumbar puncture site, this seems quite unlikely to be the cause of early renal contrast. If early renal contrast was due to contrast leakage from the puncture site rather than a CVF, it would be expected that >1 patient in the dural CSF leak group would have had early renal contrast because a dural CSF leak results in extradural contrast analogous to contrast leakage from the puncture site. Also, some patients in the control group would likely have had renal contrast on the initial CTM because a small amount of contrast leakage from the puncture site is common. The retrospective nature of the study was also a limitation due to a lack of standardization of imaging times and a lack of delayed imaging in the control group. This particularly included shorter times between intrathecal contrast injection and initial CTM in the dural CSF leak group compared with the other groups. One reason is that many of the patients with dural CSF leaks had a high pretest probability of a fast CSF leak and therefore had intrathecal contrast injected while on the CT table (as opposed to under fluoroscopy in the other groups), leading to a decreased time to the initial CT imaging. Contrast type (including the 8 patients in Study Group 3 who received concurrent normal saline ± Magnevist) and volume were not standardized. Also, relatively few patients in the dural CSF leak group had delayed CTM because leaks were usually identified on the initial CTM.
Conclusions
Early renal contrast on CTM, if present, appears to be a secondary sign of CSF leak and suggests CVF rather than a dural tear as the cause of the CSF leak. If renal contrast is present on the initial CTM in a patient with SIH, the radiologist should carefully scrutinize the study for a hyperdense paraspinal vein, and if none is found, more advanced diagnostic studies should at least be considered. The presence of early renal contrast on CTM also appears to be limited to patients with SIH, compatible with the abnormal CSF physiology in these patients. Given the limited size and retrospective nature of our single-institution study, further prospective studies with larger groups of patients are needed to validate these findings.
Acknowledgments
The authors acknowledge the assistance of Sonia Watson, PhD, in editing the manuscript.
ABBREVIATIONS:
- CTM
CT myelography
- CVF
CSF venous fistula
- SIH
spontaneous intracranial hypotension
Footnotes
Paper previously presented orally at: Annual Meeting of the American Society of Spine Radiology, February 23–26, 2017; San Diego, California.
References
- 1. Mokri B. Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache 2013;53:1034–53 10.1111/head.12149 [DOI] [PubMed] [Google Scholar]
- 2. Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology 2014;83:472–73 10.1212/WNL.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 3. Kranz PG, Amrhein TJ, Schievink WI, et al. . The “hyperdense paraspinal vein” sign: a marker of CSF-venous fistula. AJNR Am J Neuroradiol 2016;37:1379–81 10.3174/ajnr.A4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark MS, Diehn FE, Verdoorn JT, et al. . Prevalence of hyperdense paraspinal vein sign in patients with spontaneous intracranial hypotension without dural CSF leak on standard CT myelography. Diagn Interv Radiol 2018;24:54–59 10.5152/dir.2017.17220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halkar R, Pettigrew R, Lipman C, et al. . Rapid transit of tracer to the kidneys and urinary bladder in radionuclide cisternography: a sign of CSF leak. Clin Nucl Med 1997;22:121–23 10.1097/00003072-199702000-00012 [DOI] [PubMed] [Google Scholar]
- 6. Mokri B. Radioisotope cisternography in spontaneous CSF leaks: interpretations and misinterpretations. Headache 2014;54:1358–68 10.1111/head.12421 [DOI] [PubMed] [Google Scholar]
- 7. Kranz PG, Luetmer PH, Diehn FE, et al. . Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. AJR Am J Roentgenol 2016;206:8–19 10.2214/AJR.15.14884 [DOI] [PubMed] [Google Scholar]
- 8. Sage MR. Kinetics of water-soluble contrast media in the central nervous system. AJR Am J Roentgenol 1983;141:815–24 10.2214/ajr.141.4.815 [DOI] [PubMed] [Google Scholar]
- 9. Herwerth M, Prothmann S, Kreiser K, et al. . Spontaneous cerebrospinal fluid leak with venous engorgement mimicking a contrast-enhancing cervical mass. JAMA Neurol 2016;73:886–87 10.1001/jamaneurol.2016.0277 [DOI] [PubMed] [Google Scholar]
- 10. Schievink WI, Moser FG, Maya MM, et al. . Digital subtraction myelography for the identification of spontaneous spinal CSF-venous fistulas. J Neurosurg Spine 2016;24:960–64 10.3171/2015.10.SPINE15855 [DOI] [PubMed] [Google Scholar]
- 11. Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol 2017;209:1360–66 10.2214/AJR.17.18351 [DOI] [PubMed] [Google Scholar]