Fig. 6. Tumour growth studies in a PC3M-Luc-G5 murine model.
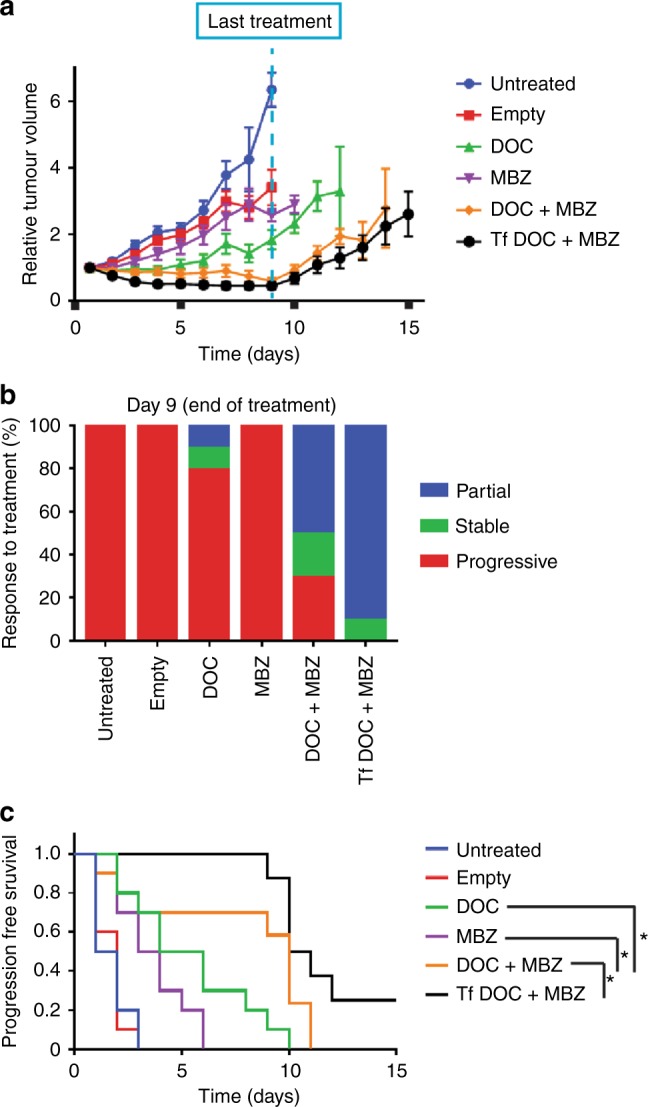
a PC3M-Luc-G5 cells were subcutaneously injected into male immunodeficient BALB/c mice, and when tumours were palpable and reached a diameter of 5 mm, the animals were randomised to receive intravenous administration of untargeted liposomes entrapping docetaxel and mebendazole (DOC + MBZ), docetaxel only (DOC) or mebendazole only (MBZ), along with control mice that were either untreated or injected with empty liposomes. In addition, a group of mice were injected with transferrin (Tf)-bearing liposomes entrapping DOC + MBZ for a focused comparison with untargeted liposomes entrapping DOC + MBZ. n = 10 (except for Tf DOC + MBZ, n = 8), mean values ± SEM are shown. b Overall tumour (n = 10) response at the end of treatment, classified in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST), where progressive disease = increase in relative tumour volume higher than 1.2-fold, stable disease = relative volume between 0.7 and 1.2 and partial response = measurable tumour with a volume reduction of more than 30% (0–0.70). c Progression-free survival curves. The y axis gives the proportion of tumours that are progressing over time. Progression-free survival was defined as < 20% increase in tumour volume from day 1. n = 10 (except for Tf DOC + MBZ, n = 8), analysed by log-rank (Mantel–Cox) test, *p < 0.05.
