Table I.
Physicochemical Properties and Structure of the Compounds Used for the Solubility Experiments (ChemDraw Professional 15)
| Drug | Ionization | Lipophilicity (log P)* | Solubility** | |
|---|---|---|---|---|
| Neutral Drugs |  |
Neutral (pKa=9.38) (28) | 0.20 (28) | High (28) |
 |
Neutral (pKa=15)a | 2.45 (29) | Low (30) | |
| Weak acids |  |
Weak acid (pKa=3.8) (31) | 2.29 (31) | Low (31) |
 |
Weak acid (pKa=4.5) (32) | 4.00 (33) | Low (32) | |
| Weak Bases | 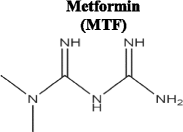 |
Weak base (pKa=2.8) (34) | -1.43 (35) | High (34) |
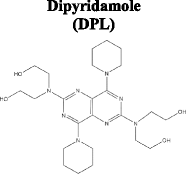 |
Weak base (pKa=6.2) (36) | 2.74 (37) | Low (38) | |
 |
Weak base (pKa=4.5) (39) | 6.20 (39) | Low (40) | |
| Ampholytes | 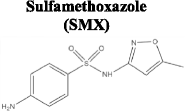 |
Ampholyte [Weak base: pKa1=1.7/Weak acid:. pKa2=5.6] (41) | 0.89a | Low (42) |
*Experimental values, ** based on the compound’s BCS (Biopharmaceutical Classification System) classification (high: BCS Class I and III; low: BCS Class II and IV) (27), aSource: DrugBank
