Figure 5.
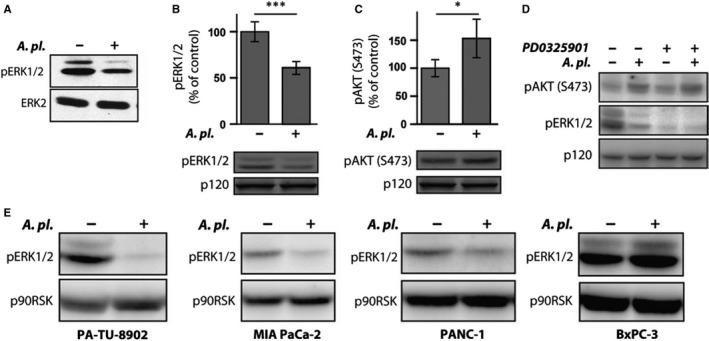
The effect of Arthrospira platensis extract on ERK and AKT activity in human PA‐TU‐8902 pancreatic cancer cells. A, A platensis suppresses ERK activation in PA‐TU‐8902 cells. Cell lysates from cells treated with A platensis extract for 1 h were probed with antibody recognizing active, doubly phosphorylated ERK (pERK1/2) to determine the activation level of ERK. In parallel, cell lysate blots were re‐probed with an ERK2 antibody to confirm equal protein loading. B, Quantification of ERK activity in A platensis‐treated PA‐TU‐8902 cells. Cell lysates from (A) run in duplicates were probed with antibody recognizing active, doubly phosphorylated ERK (pERK1/2) and IR700 dye‐labelled secondary antibody. The fluorescent signal was normalized to p120RasGap probed with IR800 dye secondary antibody. Data are plotted as mean ± SD, with the ERK activity in untreated cells as a reference. C, Quantification of AKT activity in A platensis‐treated PA‐TU‐8902 cells. Cell lysates from (A) run in duplicates were probed with antibody recognizing active AKT (pAKT‐S473) and IR700 dye‐labelled secondary antibody. The fluorescent signal was normalized to p120RasGap probed with IR800 dye secondary antibody. Data are plotted as mean ± SD, with the AKT activity in untreated cells as a reference. D, Effect of ERK inhibition on the phosphorylation of AKT in PA‐TU‐8902 cells. Cell lysates from cells treated with MEK inhibitor PD0325901 (1 μmol/L) and A platensis extract for 1 h were probed with antibody recognizing phosphorylated ERK (pERK1/2) and phosphorylated AKT (pAKT‐S473) to determine the activation level of ERK and AKT, respectively, and HRP‐labelled secondary antibody. Equal protein loading was confirmed by blotting for p90RSK. E, A platensis inhibits ERK activation in different pancreatic cancer cell lines. Cell lysates from pancreatic cancer cell lines (PA‐TU‐8902, MIA PaCa‐2, PANC‐1 and BxPC‐3) treated with A platensis extract for 1 h were probed with antibody recognizing active, doubly phosphorylated ERK (pERK1/2) and HRP‐labelled secondary antibody to determine the activation level of ERK. Equal protein loading was confirmed by blotting for p90RSK
