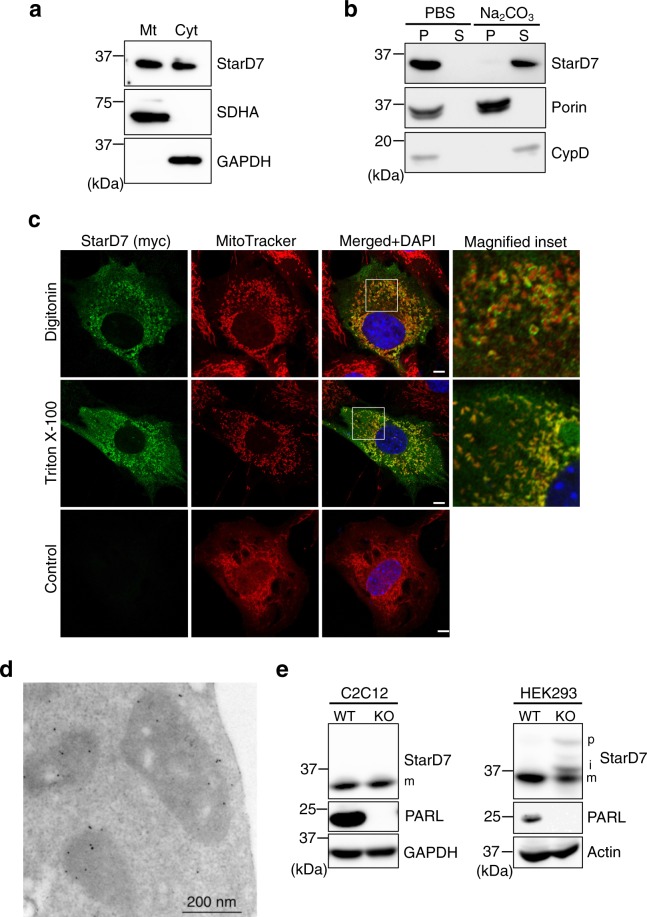
Figure 1.
Sub-mitochondrial distribution and PARL-independent maturation of StarD7 in C2C12 cells. (a) Mitochondria and cytosol were separated from C2C12 cells by subcellular fractionation and analyzed by western blotting using anti-StarD7, -SDHA and -GAPDH antibodies. Mt and Cyt indicate mitochondria and cytosol, respectively. (b) Mitochondria isolated from the cells were treated with PBS or alkaline buffer (Na2CO3), then the pellet (P) and supernatant (S) were separated by centrifugation. These fractions were analyzed by western blotting with anti-StarD7, porin and CypD antibodies. Porin is a membrane-integrated protein and CypD is a matrix protein. (c) C2C12 cells in proliferation condition were transfected with the expression vector for StarD7 fused with a myc tag at the C-terminus. Cells were permeabilized with 0.005% digitonin (w/v) or 0.1% Triton X-100 (w/v), then immunostained with anti-myc antibody (green). Control means cells without detergent treatment. Mitochondria and nuclei were stained with MitoTracker Red (red) and DAPI (blue), respectively. Bars indicate 5 μm. (d) C2C12 cells were transfected with the expression vector for StarD7 fused with a V5 tag at the C-terminus. After fixation, sections were stained with anti-V5 antibody followed by secondary gold-conjugated antibody. (e) Cell lysates from WT and PARL-KO C2C12 and HEK 293 cells were separated by SDS-PAGE, then the proteins were analyzed by western blotting using anti-StarD7 and PARL antibodies. p, precursor; i, intermediate; m, mature form of StarD7. GAPDH and actin were used as protein loading controls.