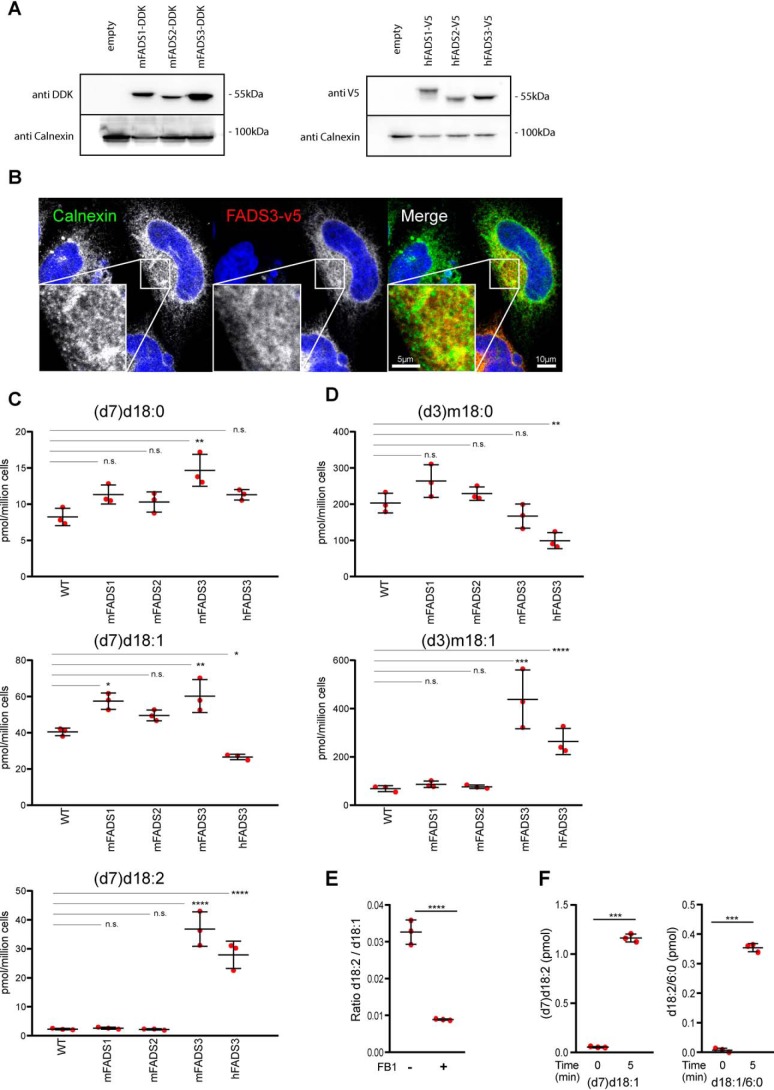
Figure 2.
FADS3 mediates the Δ14 LCB desaturation in mammalian cells. A, Western blotting of HEK293 cells stably expressing mFADS1–3 (Myc-DDK) and hFADS1–3 (V5). B, intracellular localization of hFADS3-V5 (red) overlaps with the ER marker calnexin (green). Scale bar, 10 μm. Insets, 3-fold magnification. Scale bar, 5 μm. C, cells stably expressing mFADS1–3 and hFADS3 cultured for 48 h with isotope-labeled (d7)d18:0. The levels of (d7)d18:2 were significantly elevated in mFADS3- and hFADS3-overexpressing cells but not altered in mFADS1 and mFADS2 cells. D, FADS1–3–expressing HEK293 cells supplemented with isotope-labeled (d3)m18:0 for 48 h. (d3)m18:1 levels were higher in FADS3-expressing cells compared with WT, mFADS1, and mFADS2 cells. E, HEK293 cells supplemented with (d7)d18:1 in the presence or absence of FB1 (35 μm). (d7)d18:2 formation was reduced but still detectable in the presence of FB1, indicating that FADS3 can also metabolize the free LCB. F, FADS3 in vitro activity with the free LCB ((d7) d18:1) and the N-acylated form (d18:1/6:0). The free and the N-acylated form were both rapidly converted into (d7)d18:2 and d18:2/6:0, respectively. Data are shown as mean ± S.D. (error bars), n = 3, paired t test; ***, p < 0.001; ****, p < 0.0001; n.s., not significant.