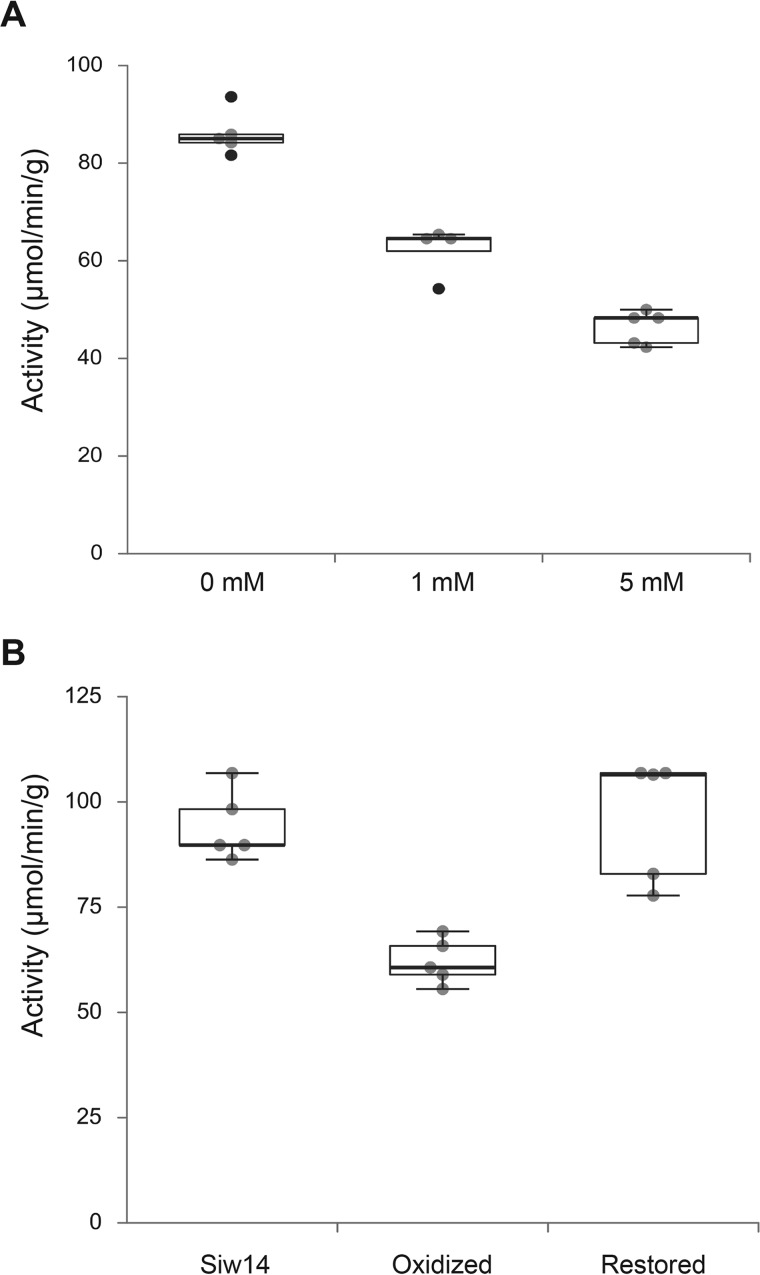
Figure 5.
Reversible inhibition of Siw14 phosphatase activity in vitro. A, recombinant His6-MBP-Siw14 protein was purified and treated with 0, 1, and 5 mm hydrogen peroxide for 30 min on ice, and assayed for phosphatase activity using p-nitrophenol phosphate as the substrate. Absorbance was measured at OD405 and activity was determined relative to a standard curve with p-nitrophenol. Activity was defined as 1 unit = 1 μmol/min/g of protein. B, recombinant, purified His6-MBP-Siw14 protein was treated with 1 mm hydrogen peroxide as in panel A, and then treated with 1 unit of catalase for 30 min on ice followed by the addition of 100 μm DTT. Phosphatase activity was measured as described above. The oxidized enzyme has enzyme activity that is different from the untreated enzyme (p = 0.005, Student's t test) and the restored enzyme is not different from the untreated enzyme (p = 0.367).