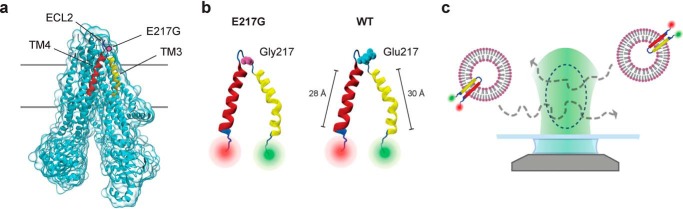
Figure 1.
a, structure of human CFTR (44) (Protein Data Bank code 5UAK) highlighting the position of the E217G mutation in the interconnecting loop ECL2 (blue) of TM3/4 (yellow/red). Visualization was generated using Visual Molecular Dynamics (VMD) (47). b, schematic representation of the E217G (left) and WT (right) TM3/4 helical-hairpin motifs comprising CFTR's TM helices TM3 (yellow) and TM4 (red) and the intervening extracellular loop ECL2 (blue). The residues at position 217 are represented as van der Waals surfaces. The lengths of TM3 and TM4 are indicated for WT TM3/4, estimated from the cryo-EM structure (44) (Protein Data Bank code 5UAK). Visualization was generated using VMD (47). c, schematic of the single-molecule FRET approach for investigating hairpin conformations. Shown are single fluorescently labeled TM3/4 hairpin molecules reconstituted into phospholipid vesicles (not to scale) freely diffusing through the observation volume of the confocal microscope.