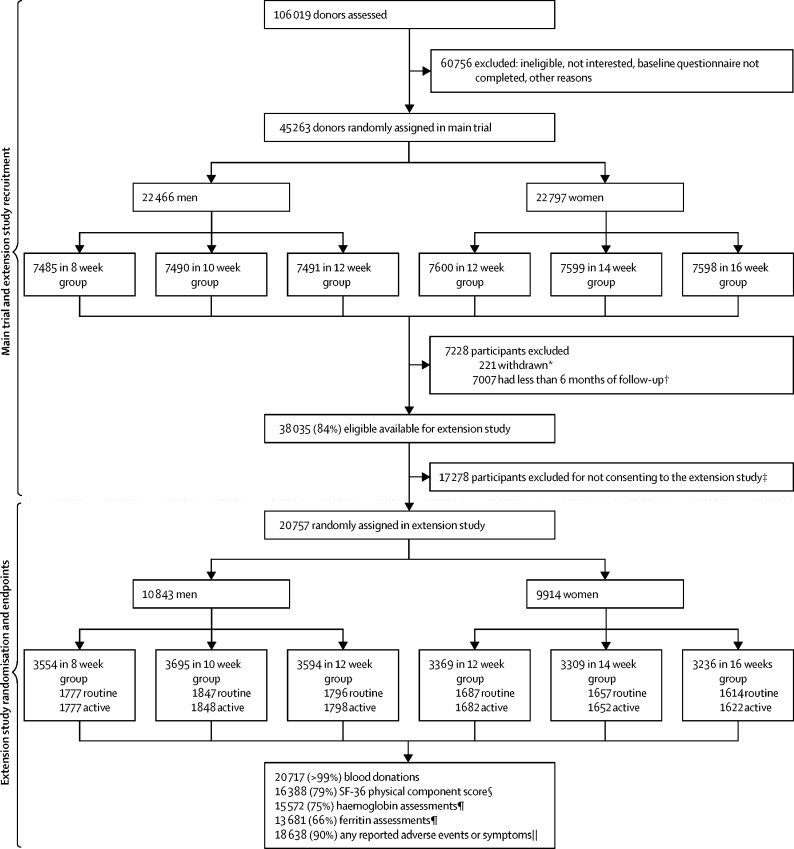
Figure 1.
Trial profile
CONSORT flowchart showing recruitment, participation, and completeness of main outcomes in the extension study. *Participants who were randomised but later withdrew consent for any further use of their data. †Due to staggered roll-out of the main 2-year trial, only participants expected to attend at least two more sessions were considered eligible for invitation to the extension study. ‡Participants not consenting to the extension study reverted to routine NHS Blood and Transplant reminders (men every 12 weeks, women every 16 weeks). §Number for whom a physical component score could be calculated at the end of the extension trial. ¶Number who provided a research blood sample at the end of the extension trial from which haemoglobin and ferritin were measured. ||Number who responded to at least one question in any of the 6-monthly questionnaires administered during their participation in the extension study.