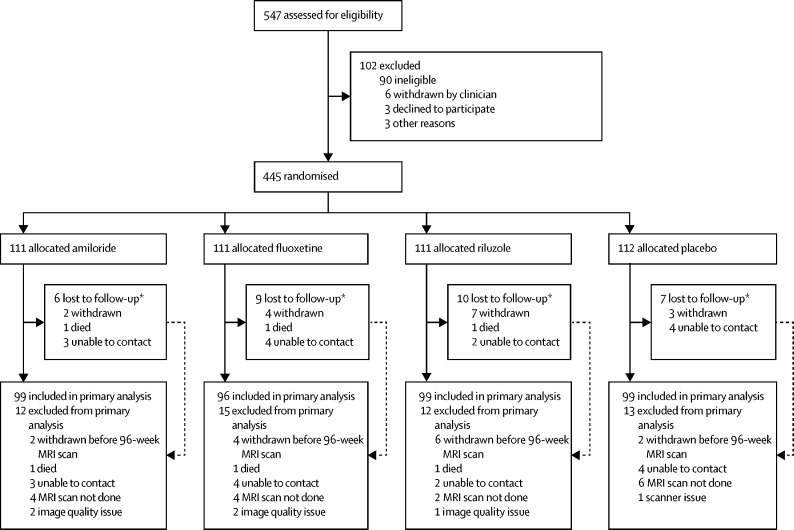
Figure 1.
Trial profile
*All patients lost to follow-up at any time during active follow-up (ie, up to and including the 100-week telephone call). Two patients withdrew after the 96-week MRI scan but before the end of the study (one allocated riluzole and one allocated placebo) and were included in the primary analysis. Two patients allocated riluzole also received fluoxetine prescribed by their family doctor towards the end of the trial. One patient allocated riluzole was withdrawn by a clinician: all other withdrawals were the patient's decision.