Figure 1.
Overview of the Chromosome Transplantation Protocol Developed to Correct Human iPSCs
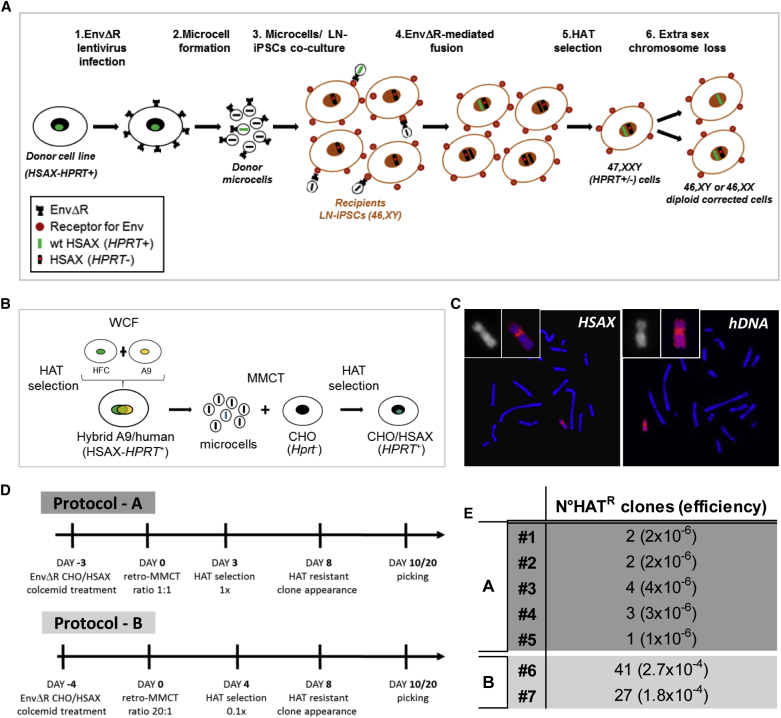
(A) Scheme illustrating the CT protocol followed to generate corrected LN-iPSCs in which an endogenous sex chromosome is replaced with an exogenous normal X chromosome (HSAX-HPRT+) to rescue the genetic defect. (1) A donor cell line containing a normal human X chromosome (HSAX-HPRT+) was infected with the EnvΔR lentivirus. (2) Microcells from the EnvΔR donor cells were obtained and (3) co-cultured with the recipient LN-iPSCs to mediate cell-microcell fusion. (4) The resulting fused cells were selected in HAT medium (5) to identify those in which the normal HSAX has been acquired. (6) Finally, corrected diploid clones in which an endogenous sex chromosome has been lost were identified. HSAX, Homo sapiens X chromosome; HPRT, hypoxanthine phosphoribosyltransferase; EnvΔR, lentivirus expressing the envelope protein of the murine leukemia viruses (MLVs); HAT medium, hypoxanthine-aminopterin-thymidine medium. (B) Schematic representation showing the procedure followed to generate the donor cell line (CHO/HSAX) containing a normal X chromosome suitable for the retro-MMCT protocol. WCF, whole-cell fusion; HFC, human fibroblast cell; HSAX, Homo sapiens X chromosome; CHO, Chinese hamster ovary; HPRT, hypoxanthine phosphoribosyltransferase; HAT medium, hypoxanthine-aminopterin-thymidine medium. (C) Representative metaphase spreads after FISH on an HAT-resistant CHO/HSAX clone using the X chromosome painting (red, left) and human genomic DNA (hDNA in red, right) as probes, respectively. (D) Time lines of the two retro-MMCT protocols here exploited (protocols A and B). (E) Efficiencies of different experiments in the generation of HAT-resistant (HATR) clones in various experiments. In protocol A, 1 × 106 recipient human iPSCs were used for every experiment; in protocol B, 1.5 × 105 recipient human iPSCs were used for every experiment.