Figure 2.
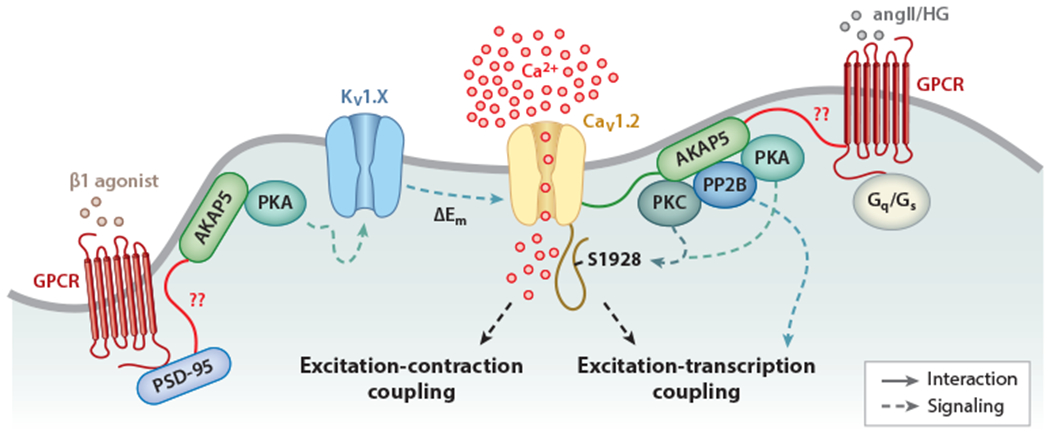
Proposed model for the regulation of vascular smooth muscle cell (VSMC) excitability by macromolecular complexes. The magnitude of Ca2+ influx via CaV1.2 is critical for the control of excitation-contraction and excitation-transcription coupling in these cells. Under physiological conditions, K+ channels oppose pressure-induced depolarization to limit CaV1.2 activity and VSMC contractility. The activity of K+ and CaV1.2 channels can be regulated by cAMP-dependent protein kinase (PKA), protein kinase C (PKC), and the protein phosphatase PP2B, which are targeted to the specific channels and G protein-coupled receptors (GPCRs) by AKAP5 and/or PSD-95, and their function may be altered during pathological conditions. Both PKA and PKC can phosphorylate CaV1.2 on S1928 (dashed blue lines) but are regulated by different GPCRs. PKA can also regulate K+ channels of the KV1 family. In turn, KV1 channels negatively control CaV1.2 activity. Whether the hypothesized interactions (solid red lines), including those involving the GPCRs that mediate angiotensin II (angII) and high glucose (HG) signaling, PSD-95, and AKAP5, occur in native VSMCs is unclear.
