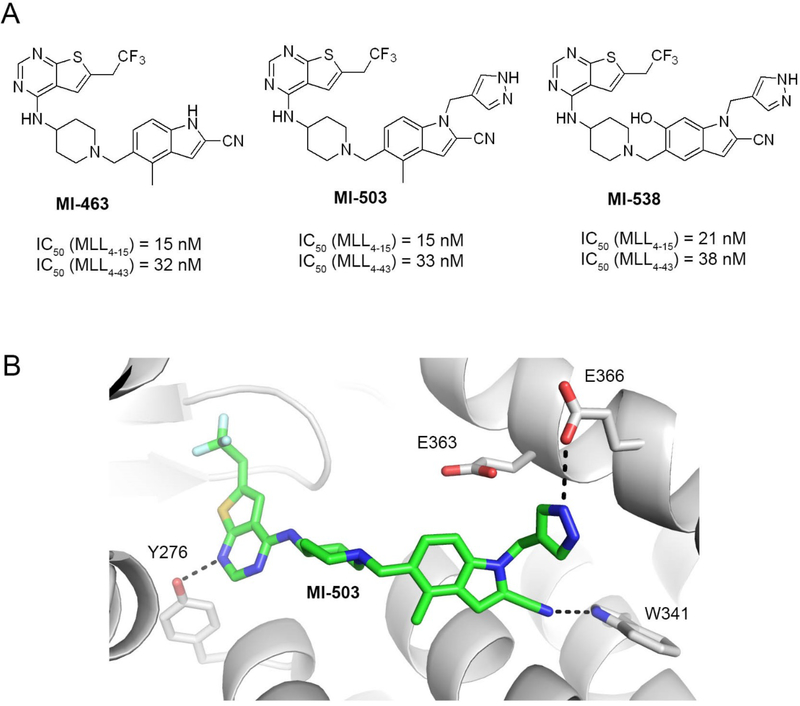
Figure 1. Structures and binding mode of the previous generation of menin-MLL inhibitors.
a) Chemical structures of the thienopyrimidine menin-MLL inhibitors MI-463, MI-503 and MI-538 together with the inhibitory activity of these compounds measured in the fluorescence polarization (FP) competition assays with menin and the fluorescein labeled MLL4–15 or MLL4–43 peptides. b) Crystal structure of menin in complex with MI-503 (4X5Y in PDB). Protein is shown in ribbon representation and key menin residues involved in interactions with MI-503 are shown as sticks. MI-503 is shown in stick representation with colors corresponding to the atom type (green: carbons, dark blue: nitrogens, yellow: sulfur, light blue: fluorine). Dashed lines represent hydrogen bonds between MI-503 and menin.