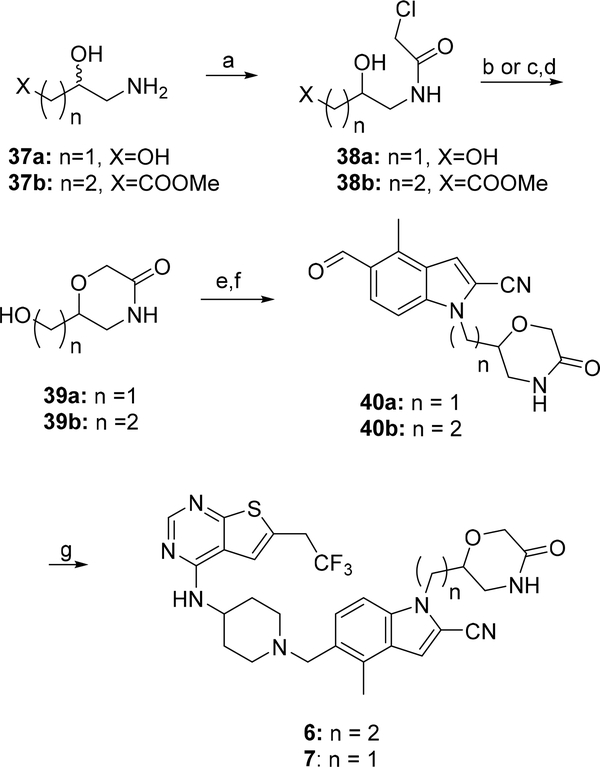
Scheme 3: Synthesis of compounds 6 and 7a.
a Reagents and conditions: a) chloroacetic acid chloride, MeOH, AcCN, TEA, 0°C; b) t-BuOK, amyl alcohol, rt (for 38a); c) LiBH4, THF, 0°C and d) t-BuOK, amyl alcohol, 0°C (for 38b); e) MsCl, TEA, DCM, −67°C- rt; f) 30, Cs2CO3, DMA, 105°C; g) 33, Na(AcO)3BH; AcOH, 50°C.