Abstract
Melanoma patients resistant to RAF/MEK-inhibitors (RMi) are frequently resistant to other therapies, such as immune checkpoint inhibitors (ICI), and individuals succumb to their disease. New drugs that control tumor growth and favorably modulate the immune environment are therefore needed. We report that the small molecule CX-6258 has potent activity against both RMi sensitive (RMS) and resistant (RMR) melanoma cell lines. Haspin Kinase (HASPIN) was identified as a target of CX-6258. HASPIN inhibition resulted in reduced proliferation, frequent formation of micronuclei (MN), recruitment of cGAS and activation of the cGAS-STING-pathway. In murine models, CX-6258 induced a potent cGAS-dependent type-I-interferon response in tumor cells, increased IFNγ-producing CD8+ T-cells and reduced Treg frequency in vivo. HASPIN was more strongly expressed in malignant compared to healthy tissue and its inhibition by CX-6258 had minimal toxicity in ex vivo expanded human tumor-infiltrating lymphocytes (TILs), proliferating TILs and in vitro differentiated neurons, suggesting a potential therapeutic index for anti-cancer therapy. Furthermore, the activity of CX-6258 was validated in several Ewing sarcoma and multiple myeloma cell lines. Thus, HASPIN inhibition may overcome drug resistance in melanoma, modulate the immune environment and target a vulnerability in different cancer lineages.
INTRODUCTION
The therapeutic options for patients with advanced or metastatic melanoma have significantly improved in the last decade. About half of melanomas harbor BRAF mutations, which sensitizes tumors to RAF/MEK inhibitors(1–5). A major limitation of these drugs is intrinsic and acquired resistance(6). For patients who respond initially and then exhibit RAF/MEK inhibitor resistance (RMR), disease progression is often rapid with reduced responsiveness to subsequent therapies, including immune checkpoint inhibitors (ICI), such as anti-CTLA-4 and/or anti-PD-1/PD-L1(7,8). In contrast to a 40–60%(9,10) response rate in the first-line setting, ICI therapy is effective in only 0–12% of RMR patients. The reasons for this observation are poorly understood at a molecular level, but it is plausible that rapid tumor growth in RMR patients outpaces the relatively slow pharmacodynamics of ICI, so that patients die before experiencing the benefits of ICIs. It seems possible that this challenge will also impact treatment of other tumor types in which oncogene-targeted and ICI therapy are currently alternative possibilities. New drugs able to control tumor outgrowth and increase the likelihood of response to ICI by inducing a favorable immune environment could therefore be beneficial.
An emerging therapeutic strategy in the treatment of multiple types of cancer is the use of inhibitors of cell cycle regulators, such as cyclin dependent kinases (CDK) and Aurora kinase in conjunction with immunotherapy. CDK4/6 inhibitors, for example, enhance anti-tumor immunity by increasing responsiveness to ICIs and/or by activation of NK cells(11,12). PARP and Aurora kinase inhibitors, activate the DNA damage response machinery and may trigger cytosolic DNA sensing via cGAS-STING resulting in expression of type I interferon response(13). This may, in turn, promote an immunogenic tumor environment that is favorable to immunotherapy. However, some of these agents, such as Aurora kinase inhibitors, have significant off-target activity and their clinical use may be limited by toxicity(14).
In this study, we identify a small molecule (CX-6258) that overcomes resistance to RAF/MEK inhibitors in melanoma cell lines. CX-6258 is annotated as an inhibitor of the PIM kinase family(15) but we find that it is primarily a potent inhibitor of the Histone H3 associated protein serine/threonine kinase (HASPIN), an understudied kinase (16). HASPIN but not PIM1–3 inhibition triggers a cascade of DNA damage, micronuclei formation and activation of cGAS-STING, resulting in type I interferon expression in tumor cells. As a result, the immune microenvironment is depleted of immunosuppressive T-regulatory cells and there is an increase in IFNγ producing CD8+ T cells. We find that HASPIN inhibition is a vulnerability in other cancers, including multiple myeloma and Ewing sarcoma, and we demonstrate activity of CX-6258 in these settings. We propose that HASPIN inhibition may be a feasible therapeutic strategy in RMR melanoma and other tumor lineages by mediating anti-tumor activity through both, cell-intrinsic mechanisms and modulation of the immune microenvironment.
METHODS
Cell lines
A375 were cultured in DMEM (Gibco® Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco). UACC62 were cultured in RPMI 1640 with 10% FBS. Braf/Mek-inhibitor resistant cell lines were generated by culturing Braf/Mek-inhibitor sensitive cell lines in 10 nM Dabrafenib and 1 nM Trametinib (A375) or 7.5 nM Dabrafenib and 0.75 nM Trametinib (UACC62) until resistant clones emerged.
The murine cancer cell line CT26 was from ATCC and was cultured in RPMI 1640 with 10% FBS.
Human myeloma cell lines AMO1, NCI-H929, SK-MM-1, U266, JJN3 and KMS-12-BM were purchased from DSMZ (Braunschweig, Germany). KMS-20 were kindly provided by Dr. K.C. Anderson (Dana-Farber Cancer Institute). These cells were cultured in RPMI-1640 medium supplemented with 10% FBS (Lonza) and 1% penicillin/streptomycin. The IL-6 dependent cell line XG-1, kindly provided by Dr. Renate Burger (University of Erlangen-Nuernberg, Erlangen, Germany), was cultured in the presence of 2.5 ng/mL rhIL-6 (R&D Systems, Minneapolis, MN). Ewing’s sarcoma cell lines RDES, SK-ES-1 and SK-NEP-1 were obtained from ATCC. SK-ES-1 and SK-NEP-1 cells were cultured in McCoy’s 5A Modified Medium (Gibco), supplemented with 15% FBS (PAN-Biotech). RDES cells were cultured in RPMI 1640 medium 10% FBS.
Cells were STR (short tandem repeats) authenticated. All cell lines were grown at 37°C, 5% CO2 in antibiotic free media and repeatedly tested negative for mycoplasma using PlasmoTest (InvivoGen).
ATP assay
Melanoma cells were seeded at 2×104 cells per well of a 96-well plate, allowed to adhere over-night and then treated with 1 μM (screen) or a dilution series of the drugs indicated, with 6 wells per concentration/condition. 72h – 96h after drug treatment half of the cell culture media was removed and 50 μL of Cell Titer-Glo (CTG) assay reagent (Promega, Madison, WI, USA) was added. Luminescence was detected after 10 min incubation on a Perkin Elmer Envision Plate reader (Perkin Elmer, Waltham, MA, USA).
Human myeloma cell lines were cultured in suspension using12-well plates (105cells/well; final volume 2mL) in the presence of CX-6258 or DMSO as control. After 72 hours, cell viability was evaluated by CTG. For CTG assay, 50μL from each experimental point were plated in triplicates in 96-well plates; then, 50μL of CTG were added and luminescence was detected by SpectraMax M3 (VWR, Radnor, PA, USA), according to manufacturer’s instructions.
To perform viability assay on Ewing sarcoma cell lines, RDES and SK-ES-1 cells were seeded at a density of 15,000 cells per well, and SK-NEP-1 at a density of 5,000 cells per well, in 4 replicates in black opaque 96-well plates, with complete medium containing either the drug or an equal volume of solvent (DMSO). After 96 hours, CTG was added, and luminescence was measured with a SynergyMx instrument (BioTek, Winooski, VT, USA). All values are reported as % of the signal obtained with control cells treated with DMSO only. Dose-response curves were fitted to the data using GraphPad Prism 8.0 (GraphPad, San Diego, Ca, USA) using a non-linear fit with variable slope (four parameters).
In vitro measurement of kinase inhibitory activity
CX-6258 was assayed using the KINOMEscan® assay platform (DiscoverX, Fremont, CA, USA). Data are reported as percent of remaining activity at 100 nM drug concentration. The activity of CX-6258, SGI1776, AZD1208, and PIM-447 on PIM1, PIM2, PIM3, Haspin and MYLK4 were assayed using Reaction Biology Corporation’s (Malvern, PA, USA) Kinase Profiling services as described previously(17). IC50 values were obtained from 10-point dose curves (3-fold dilutions with a maximum concentration of 1 μM). An ATP concentration of 10 μM was used. All compounds were obtained from Selleckchem (Houston, TX, USA), and tested for identity and purity by LC/MS in house as described in detail in the drug collection section of the HMS LINCS Database (http://lincs.hms.harvard.edu/db/sm/).
Measurement of cell viability and cell cycle perturbation
Cells were seeded and treated as indicated above. Cells were stained and fixed for analysis at the time of drug delivery and after 48–96 hours of incubation roughly equivalent to two doubling times of each cell line. Cells were pulsed for one hour with EdU (Lumiprobe, Hunt Valley, MD, USA) and stained with 1:2000 LIVE/DEAD Far Red Dead Cell Stain (LDR) (ThermoFisher, Waltham, MA, USA), fixed with 3.7% formaldehyde for 30 minutes and permeabilized with 0.5% Triton X-100 in PBS. The EdU was labeled with cy3-azide for 30 min. The cells were then blocked for one hour with Odyssey blocking buffer, and stained overnight at 4˚C with 2 μg/ml Hoechst 33342 and a 1:1000 dilution of anti-phospho-histone H3 (pHH3) Alexa 488 (Ser10, clone D2C8) conjugated antibody (CST) and imaged using a 10X objective on a Operetta microscope (Perkin Elmer, Waltham, MA, USA) and analyzed using Columbus software (Perkin Elmer). DNA content, defined by the total Hoechst intensity within the nuclear mask, was used to identify cells in the G1 and G2 phases of the cell cycle. The average LDR, EdU and phospho-histone H3 intensities within the nuclear masks were determined. The LDR signal was used to classify cells as live or dead, the EdU and pHH3 signals to identify S and M phase cells respectively. Cells with intermediate DNA content and no EdU signal were classified as S phase dropout cells. Live cell counts were normalized to DMSO-treated controls on the same plates to yield normalized growth rate inhibition (GR) values as described previously(18).
Life cell cell-cycle imaging
A375 reporter cells stably expressing H2B-Venus and mCherry-geminin(1–110) were imaged as previously described (19). Details are described in Supplemental Data.
CRISPR/Cas9 mediated genome editing
Recombinant Cas9 protein (Makrolab, Berkley, CA, USA) complexed with crRNA (Integrated DNA Technologies, Coralleville, IA, USA) was introduced into the tumor cell lines by electroporation using Program FF-120 with SF-Buffer on an Amaxa 4D-Nucleofector (Lonza,). The guide RNA sequences were: hPIM1 [AGAAGGACCGGATTTCCGAC], hPIM2 [CACTCGAAGTCGCACTGCTA], hMYLK4 [GTGGTCAAACGCCGACCTGA], mCgas [GCGAGGGTCCAGGAAGGAAC]. A guide targeting LacZ [GCTGAGCGCTCGGAGCGCCT] served as control.
Short interfering RNA (siRNA) mediated gene knock down
Previously validated siRNA targeting Haspin (Ambion Silencer Select ID 38320 and 38321, ThermoFisher) or negative controls were transfected into A375 cells using Lipofectamine® RNAiMax Transfection Reagent (ThermoFisher) using the manufacturers recommended protocols.
Western blotting
Cell culture pellets were collected by scraping down the cells into ice cold PBS. After centrifugation, pellets were either stored at −20C until further processing or immediately lysed using RIPA Buffer (Sigma-Aldrich, St. Louis, MO, USA) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail for 30 minutes on ice. Samples were then micro-centrifuged at 12,000 g to remove debris. Protein concentration of the lysate was estimated using BCA assay using Pierce BCA Protein Assay Kit (ThermoFisher). Equal amounts of protein were boiled with 6x SDS-sample Buffer (Boston BioProducts, Boston, MA, USA) for 10 minutes and loaded on 10% TGX stain-free FastCast Acrylamide gels (Bio-RAD, Hercules, CA, USA). Protein was transferred to PVDF membranes, blocked for 1 h at room temperature with 5 % milk in TBS-T and the membranes were probed with primary antibodies in 5% BSA overnight. Primary antibodies were procured from the following manufacturers: PIM-1(D8D7Y); PIM-2(DID2); PIM-3(D17C9); Phospho-BAD (Ser 112) (40A9); CHK2(D9C6); Phospho-CHK2 (Thr68) (C13C1); p53 (1C12); Phospho-p53 (Ser 392); STING (D1V5L); Phospho-STING (Ser 365) (D8F4W); CGAS (D1D3G), (D3O8O); MEK-1/2; Phospho- MEK-1/2(Ser 217/221); ERK1/2 (3A7); Phospho- ERK1/2 (Thr202/Tyr204) and Vinculin – Cell Signaling Technology Inc. (Danvers, MA, USA), Haspin (ab226222) was obtained from abcam (Cambridge, UK). After washing, the membranes were probed with Anti-rabbit IgG and Anti-mouse IgG HRP-linked secondary antibodies (Cell Signaling) and imaged on a Licor Odyssey Fc Imaging System. Equal protein loading was assessed using actin or vinculin antibodies and fluorescent detection.
qPCR
Total RNA was isolated using RNeasy plus kit (Qiagen, Hilden, Germany) and 500 ng RNA per 20 μL reaction was transcribed into cDNA using iScript Supermix (BioRad). Taqman assays (ThermoFisher) for Ifna1 (#Mm00439552_s1) and Ifnb1 (#Mm03030145_gH) were run on a QuantStudio 6 Flex (Applied Biosystems) with TaqMan™ gene expression MasterMix (ThermoFisher). Gapdh (Mm99999915_g1) and Actb (Mm00607939_s1) were used as reference genes for normalization. All expression values were calculated using ΔΔCT and are reported as relative expression values.
Immunofluorescence
For immunofluorescent studies cells were grown on black walled 96 well plates (Corning, Corning, NY, USA) and treated as indicated. For staining of phosphor-H2A.X, Centromeres (CREST), tubulin, or cGAS cells were fixed and permeabilized as described above. After blocking for 1h at room temperature with Odyssey blocking buffer (Li-cor, Lincoln, NE, USA) cells were stained overnight at 4˚C with a 1:400 dilution of rabbit anti-phospho-H2AX.X S139 (9718, Cell Signaling), or a 1:1000 dilution of human anti-CREST (HCT-0100, ImmunoVision, Springdale, AR, USA) and a 1:400 dilution of rabbit anti-beta-tubulin (2128, Cell Signaling), or a 1:200 dilution of rabbit anti-cGAS-antibody (D1D3G, Cell Signaling). After washing cells were stained with secondary anti-Rabbit Alexa488 or anti-human Alex647 (ThermoFisher). After washing nuclei were stained using 2 μg/ml Hoechst 33342 (Sigma Aldrich) for 1h at RT.
Representative images were taken with an Olympus IX72 (Olympus, Tokyo, Japan) equipped with a 20x objective and Orca Spark camera (Hamamatsu, Boston, MA, USA) operated with CellSens software or selected from plate scans with a GE IN Cell Analyzer 6000 (GE Healthcare, Chicago, IL, USA). For quantification purposes plates were scanned using a GE IN Cell Analyzer 6000 and cGas spots, micronuclei, and γ-H2AX spots were quantified from 5 random fields per well using the Columbus image data storage and analysis system (Perkin Elmer).
Animal experiments
All animals used were ordered from Jackson laboratory (Bar Harbor, ME, USA). Tumor size was measured at least twice per week using a digital caliper and tumor volume was calculated using the formula V = (W2 × L)/2, with W being the shorter, and L being the longer diameter.
For A375 Xenograft studies 6-week-old female N:J mice were subcutaneously injected with 2.5×106 cells per mouse in a 1:1 PBS/Matrigel mixture. Treatment was administered by oral gavage when tumor became palpable and mice were treated for five days with 100 mg/kg body weight (bw) CX-6258 dissolved in water.
For CT26 studies in N:J mice 3×105 cells per mouse were injected in PBS subcutaneously, and treatment was administered by oral gavage when tumors became palpable. Mice received treatment via oral gavage on five consecutive days with doses of either 10 mg/kg bw or 100 mg/kg bw CX-6258.
For syngeneic CT26 studies 6–8-week-old female Balb/c mice were subcutaneously injected with 4×105 cells per mouse in PBS. On day 5 to 7 treatment was initiated when tumors were palpable. The CX-6258 only group received either 10 mg/kg bw or 100 mg/kg bw CX-6258 per oral gavage for three or four consecutive days respectively. The anti-PD1 only group received three intraperitoneal injections of 200 μg anti-PD1-antibody (29F.1A12, InVivoMab, BioXcel, New Haven, CT, USA) on days 5, 8, and 11 or 7, 10 and 13 as indicated in the respective experiment. The phased therapy group received anti-PD1 at the same days as the anti-PD1 only group but received an additional three days of 10 mg/kg bw or four days of 100 mg/kg bw CX-6258 per oral gavage from day 8 or 10 onwards. Control animals received isotype control antibody. For survival analysis, endpoints were defined as >700 mm3 tumor volume or central ulceration and necrosis. All animal studies were approved under animal protocol 08–049 at Dana-Farber Cancer Institute.
Immunophenotyping
To characterize the immune-response in tumor bearing mice from above experiment mice were treated as described above. On day 16 mice were sacrificed and spleens, tumor draining lymph nodes and tumors were harvested, and single-cell suspensions were generated by mechanical and enzymatic digestion. Dead cells were labeled using Zombie NIR (Biolegend, San Diego, CA, USA) in PBS for 15 minutes at room temperature. Thereafter surface antigens were stained in PBS supplemented with 3% FBS and 2mM EDTA for 30 minutes on ice using anti-CD45-BV510 (30-F11, Biolegend), anti-CD3-PE-Cy5 (145–2C11, Biolegend), anti-CD4-BV605 (RM4–5, Biolegend), anti-CD8a-BV785 (53–6.7, Biolegend), anti-NKp46-PE (145–29A1.4, Biolegend), anti-CD14-APC (Sa14.2, Biolegend), anti-CD45R-APC (RA3–6B2, Biolegend), anti-F4/80-APC (BM8, Biolegend).
Cells were fixed using the eBioscience™ Foxp3 / Transcription Factor Staining Buffer (ThermoFisher) as recommended by the manufacturer. Intracellular FOXP3 was stained using anti-FOXP3-BV421 (MF-14, Biolegend). Ki67 was stained using anti-Ki67-FITC (SolA15, eBioscience, San Diego, CA, USA).
For staining of intracellular IFNγ TILs isolated from tumors were seeded in 96 well round bottom plates in RPMI supplemented with 10% FBS, GlutaMax, 10 mM HEPES, and 55 μM beta-Mercaptoethanol. Cells were stimulated for 4 hours in 37 C and 5% CO2 using a combination of phorbol 12-myristate-13-acetate (PMA) and Ionomycine (BioLegend) and washed once with PBS. Viability and surface antigens were stained as described above. Cells were fixed using the eBioscience™ Foxp3 / Transcription Factor Staining Buffer (ThermoFisher) and intracellular IFNγ was stained using anti-IFNγ-PerCp-Cy5.5 (XMG1.2, Biolegend)
Cells were acquired with a Sony SP6800 Spectral Flow Cytometer (Sony, Tokyo, Japan) and the data were analyzed using FlowJo 10.5.3 (TreeStar, Ashland, OR, USA). Fluorescence minus one (FMO) gating controls were used for FOXP3, Ki67 and IFNγ.
Toxicity assessment in human Tumor infiltrating lymphocytes
Human Tumor infiltrating lymphocytes (TILs) from melanoma patients were cultured and expanded as previously described(20). To assess cytotoxicity of CX-6258 on human TILs, cells were seeded in RPMI with 10% human AB serum, 3000 IU/ml hIL2 at 1×105 cells per well of a 96 round bottom plate (Corning) and treated with increasing doses of CX-6258 in triplicates. After 48 hours cells were collected, washed once with PBS and resuspended in Annexin V staining buffer. Cells were stained with Annexin V-FITC and Propidium iodide (PI) (Cell Signaling) and analyzed on a Sony SP6800 Spectral Flow Cytometer. Data were analyzed using FlowJo 10.5.3 (TreeStar) and viable cells defined by negative staining for Annexin V and PI are reported. For proliferation assays 5×104 TILs were stained with CFSE (Invitrogen) and seeded together with anti-CD3/CD28 Beads (Invitrogen) and increasing doses of CX-6258 in T-cell media supplemented with 30 IU/ml IL2. Cells were collected on days 1,3 and 5, stained for viability using Zombie UV dye (BioLegend) and fixed using 4 % formaldehyde in PBS. Cells were analysed by FACS on a BD-Fortessa (Becton Dickinson, Franklin Lakes, NJ, USA). Data analysis was performed with FlowJo 10.5.3 (TreeStar) and viable cells were defined by negative staining for Zombie and analyzed for CFSE intensity.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software). For experiments with two groups Student’s t-test was used. For comparison of three or more groups variance was assessed using ANOVA and significance was calculated using Tukey post-hoc test correcting for multiple comparison and adjusted p-values are reported. Tumor growth curves were compared using Two-way ANOVA or mixed effects analysis with Tukey post-hoc test comparing treatment means. Survival was analyzed using a log-rank test. P values <0.05 were considered significant.
RESULTS
Inhibition of HASPIN shows activity in RAF/MEK inhibitor sensitive and resistant melanoma
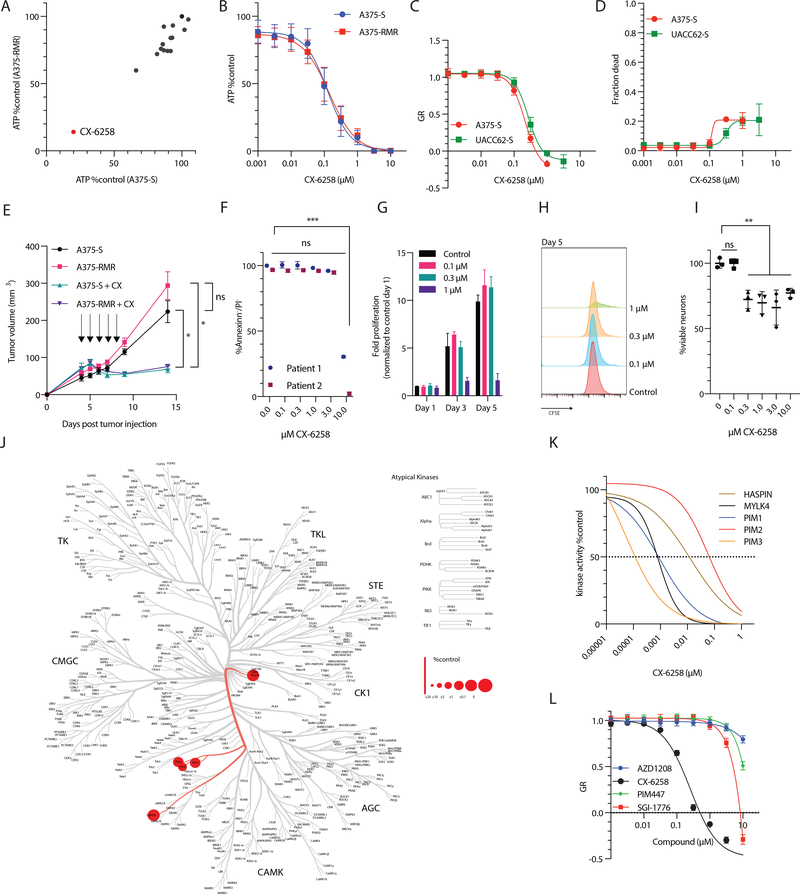
To model RMR, we used two RMI-sensitive human BRAFV600E melanoma cell lines (A375-S and UACC62 –S) and generated resistant lines (A375-RMR and UACC62-RMR) by continuous culture in the presence of the RAF-inhibitor dabrafenib and MEK-inhibitor trametinib (Supplementary Figure 1A). As expected, ERK was inhibited in RMS lines in the presence of RAF/MEK inhibitors and active in RMR lines (as assayed by pERK levels); RMR cells grew at a similar rate to RMS cell lines, and required significantly higher doses of dabrafenib or trametinib to inhibit proliferation (Supplementary Figure 1B–G). We then screened a focused 14-member small molecule library (Supplementary Table 1) consisting of compounds reported to inhibit proteins in the JAK/STAT pathway, a previously reported putative vulnerability in RMR(21). Both S and RMR cell lines were treated with 1 μM drug for three days and viable cell numbers were inferred from ATP levels in cell extracts; by this measure, CX-6258 was highly active in both A375-S and A375-RMR cells, as well as UACC62-S and UACC62-RMR (Figure 1A and B, Supplementary Figure 1H, EC50 ~100 nM in A375 and ~300 nM in UACC62). When we corrected for differences in A375 and UACC62 proliferation rates using the normalized growth rate inhibition (GR) metric(18), CX-6258 was equally potent in both cell lines with GR50 ~200 nM and GRmax ~1 μM (Figure 1C–D).
Figure 1:
(A) Summary of drug screen. Relative viability of melanoma cell lines A375-S (sensitive to RAF/MEK inhibitors) and A375-RMR (resistant to RAF/MEK-inhibitors) treated with a focused drug library (Supplementary Table 1). The x axis indicates the % viability of A375-S treated with the drug library compared to DMSO treatment; y axis indicates the % viability of A375-RMR treated with the drug library compared to DMSO treatment. Each compound in the library is indicated by a dot with a different color. CX-6258 is labeled separately. (B) Dose response curve of A375-S and A375-RMR. CX-6258 was given at doses indicated in x axis and viability was measured using ATP. The y axis indicates % viability of CX-6258 treated cells vs. DMSO treated cells. (C) Growth rate (GR) corrected effect of CX-6258 at different doses, x axis indicating μM of CX-6258, y axis indicating GR response of A375-S and UACC52-S and (D) fraction of dead cells withy axis indicating fraction of dead cells compared to vehicle treated cells. (E) Nude mice (n=5/group) were injected with A375-S or A375-RMR and treated with either CX-6258 or vehicle at days indicated by arrows. x axis indicates days since tumor injection, y axis indicates tumor volume (in mm3). Animals treated with CX-6258 had significantly smaller tumors compared to vehicle treated animals (n=5/group, Error bars, mean ± SEM. A375-S vs. A375-S + CX-6258, p= 0.02; A375-RMR vs. A375-RMR + CX-6258, p = 0.01, 2way ANOVA). (F) Annexin/PI staining of two human ex vivo expanded tumor-infiltrating lymphocytes (TILs) from melanoma Patient 1 and Patient 2 treated with DMSO or increasing doses of CX-6258 as indicated on the x axis; y axis shows the % of cells that are negative for both annexin and PI. (Error bars, mean ± SD. (***) adjusted p<0.001, ANOVA. (G) Proliferation of ex vivo expanded human tumor infiltrating lymphocytes (TILs) stimulated with CD3/CD28 in the presence of increasing doses of CX-6258. X axis days after stimulation, y axis fold expansion relative to control cells on day 1. Mean±SD. (H) Histograms of CFSE intensity dilution on day 5 of TILs compared to CFSE intensity on day 1, x axis indicating CFSE intensity (I) Human in vitro differentiated neurons were treated with CX-6258 at doses indicated on x axis, y axis indicating relative cell counts. Error bars, mean ± SD. DMSO vs. 0.1 μM, adjusted p-value > 0.99; DMSO vs. 0.3–10 μM, adjusted p-value=0.03–0.001, ANOVA). (J) Human kinome screen for CX-6258. The tree represents different families of human kinases. Red circles indicate kinases that are bound by CX-6258. The size of the circles indicates the binding of the compound relative to controls. (K) Dose-response inhibition for indicated targets by CX-6258 in vitro, x axis indicating molarity of CX-6258 and y axis indicating kinase activity in vitro. The dotted line represents IC50. (L) GR dose response in A375-S testing three functionally related compounds AZD1206, PIM447 and SGI1776 compared to CX-6258, x axis indicating doses in μM, y axis indicating GR response.
To assess activity in vivo, we generated xenografts of A375-S and A375-RMR in nude mice (n=5/group) and treated animals for five days with either vehicle control or CX-6258 (100 mg/kg) by daily oral gavage. CX-6258 significantly reduced tumor growth in both types of tumor (A375-S vs. A375-S plus CX-6258; p= 0.02; A375-RMR vs. A375-RMR plus CX-6258, p = 0.01, by 2-way ANOVA) demonstrating oral bioavailability (Figure 1E). Nude mice treated with CX-6258 did not display weight loss suggesting in vivo tolerability of the compound (Supplementary Figure 1I).
To test the impact of CX-6258 on immune cells we generated two independent cultures of patient-derived tumor-infiltrating lymphocytes (TILs) by ex vivo expansion. We then treated TILs with increasing doses of CX-6258 and measured cell viability by flow-cytometry using Annexin/PI staining. At the GR50 dose in melanoma cells of ~200 nM, both TIL cultures had >98% viability (Figure 1F); a significant reduction in viability was observed only at doses > 15 x GR50 (>3 μM). To asses if CX-6258 impairs proliferation of TILs we stimulated human TILs with anti-CD3/CD28 beads in the presence of CX-6258. At concentrations effective in cancer cells, proliferation was not impaired (Figure 1G and H, Supplementary Figure 1J) suggesting a therapeutic window. To assess potential toxicity in other cell types, we treated in vitro differentiated human neurons with CX-6258. Cell counts were not reduced in cells treated with CX-6258 at 100 nM compared to DMSO treated cells (mean difference 0.04%, adjusted p>0.99 ANOVA) while doses of 300 nM to 10 μM resulted in modest reduction of cell numbers (Figure 1I). Thus, CX-6258 is active against melanoma cells and modestly toxic against human TILs and neurons in vitro.
CX-6258 acts as a HASPIN inhibitor
CX-6258 is annotated as an inhibitor of PIM-kinases(15), a family of kinases that act downstream of the JAK/STAT pathway. As kinase inhibitors frequently exhibit target promiscuity, we aimed to identify kinase targets bound by this small molecule in an unbiased fashion and we used the KINOMEscan® assay (DiscoverX) to determine its affinity for 468 human kinases. As previously described, CX-6258 bound PIM1–3 but also bound MYLK4 and HASPIN with high affinity (Figure 1J, Supplementary Figure 1K). When we compared CX-6258 to three related compounds (AZD1206, PIM447 and SGI1776) using in vitro kinase assays we observed IC50 values ranging from pico- to nano-molar for PIM1/2/3, MYLK4, or HASPIN (Figure 1K and Supplementary Figure 1L–O). Despite having partially overlapping target spectra, GR50 values for AZD1206, PIM447, and SGI1776 in A375 were ~30-fold higher (> 3 μM) than CX-6258 (Figure 1L) demonstrating that CX-6258 has higher activity in cell-based assays.
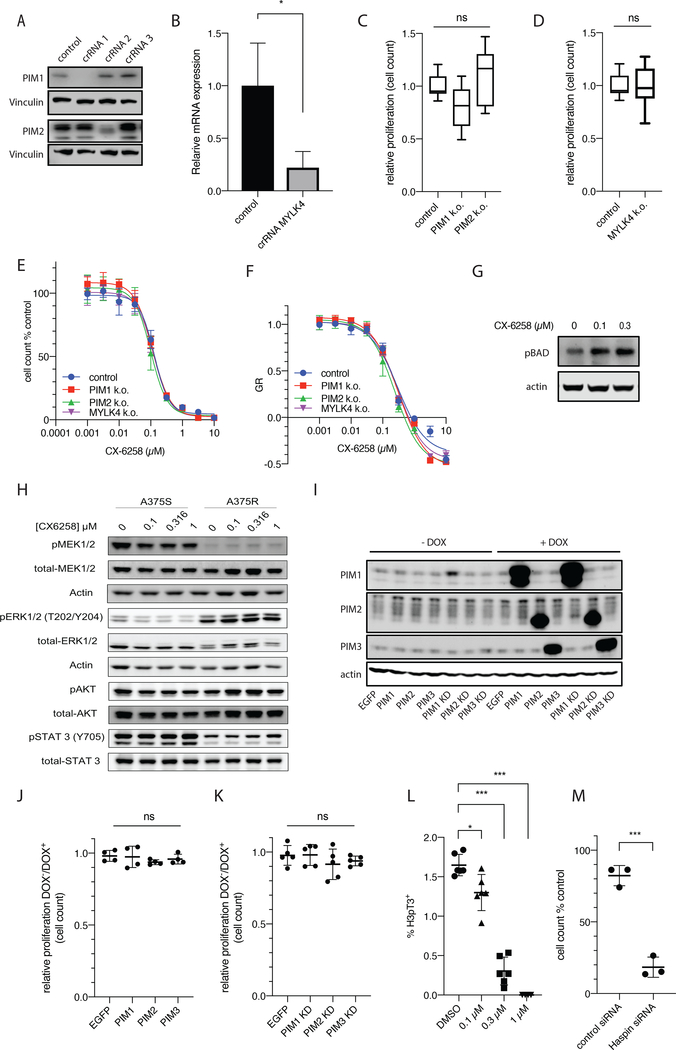
To investigate the relevance of CX-6258 targets for its biological activity we generated a series of CRISPR-Cas9 knock-outs (KO) of PIM1, PIM2 and MYLK4; knockdown was confirmed by Western Blot (for PIM) or qPCR (for MYLK4, reduced expression −77.9%, p=0.0154, t-test) respectively (Figure 2A and B). As compared to control lines electroporated with a non-targeting guide RNA (LacZ), there was no significant difference in proliferation rates for cells with KO in PIM1 (relative proliferation rate = 0.8, adjusted p value = 0.08, one-way ANOVA), PIM2 (relative proliferation rate=1.1, adjusted p value =0.47, one-way ANOVA) or MYLK4 (relative proliferation rate=1; Figure 2C and D). We were unable to establish a KO for PIM3. Furthermore, none of these KOs altered GR values for CX-6258 suggesting that PIM kinases and MYLK4 are not the relevant cellular targets of CX-6258 in melanoma cells (Figure 2E and F). Consistent with a PIM kinase-independent mode of action, exposure of cells to CX-6258 did not reduce the levels of BAD phosphorylation, a known downstream target of PIM kinases suggesting that PIMs are not the cellular target of CX-6258 or that other pathways compensate for the loss of PIM activity in melanoma cells (Figure 2G).
Figure 2.
(A) Western blots of PIM-1 and PIM-2 CRISPR-Cas9 KO. Cells were electroporated with ribonucleotides of Cas9 protein with indicated crRNAs or LacZ controls. (B) Relative mRNA expression of MYLK4 (y axis) in isogenic A375-S MYLK4 CRISPR-Cas9 KO vs. LacZ (p=0.0154, t-test). (C and D) Proliferation rate of PIM-1-KO and PIM-2-KO, and MYLK4-KO from (A) and (B) compared to their isogenic LacZ control cell lines (PIM1KO relative proliferation rate = 0.799, adjusted p value = 0.08 one-way ANOVA; PIM-2KO relative proliferation rate = 1.10, adjusted p value =0.47, one-way ANOVA). (E) Dose response of PIM-1-KO, PIM-2-KO, and MYLK4-KO cell lines from (A) and (B) to CX-6258 at doses (x axis, molarity) and in LacZ controls, with y axis indicating % cell number of CX-6258 treated cells compared to DMSO treated cells. (F) GR50 in response to CX-6258 (x axis, molarity) for all cell lines shown in E. The y axis indicates growth rate inhibition (G) Western blot of phosphorylated BAD (pBAD) and actin loading control in A375S cell lines treated with CX-6258 at indicated doses. (H) Western blot of total MEK1/2 and pMEK1/2, total ERK1/2 and pERK, total AKT and pAKT, and total STAT3 and pSTAT3, and actin controls, in A375-S and A375-RMR following treatment with indicated doses of CX-6258. (I) Western blots of PIM-1, PIM-2 and PIM-3 and actin loading controls in A375-S transduced with doxycycline-inducible vector for EGFP or ORFs for PIM-1, PIM-2, PIM-3 or kinase dead (KD) PIM-1-KD, PIM2-KD or PIM3-KD with (+Dox) or without (-Dox) doxycycline treatment. (J and K) Proliferation rate of A375-S overexpressing PIM-1, PIM-2 or PIM-3 or matched KD cell lines compared to EGFP-overexpressing cells relative to parental controls with +Dox (p=0.56 and 0.47, ANOVA). (L) Frequency (in % of all cells) of cells with positive Histone-3 phospho-Threonine 3 (H3pT3) staining (y axis) in A375-S cells treated with DMSO or indicated doses of CX-6258 showing a significant, dose-dependent reduction in response to CX-6258 treatment (Error bars, mean ± SD, p-value<0.001, linear trend significant, ANOVA). (M) Fraction of cell numbers in % of parental A375 cells (y axis) following treatment with a non-targeting siRNA or HASPIN targeting siRNA 48 hours after transfection (Error bars, mean ± SD. p-value<0.0001, t-test).
To investigate possible off-target activities of CX-6258 on pathways known to be important for the proliferation of melanoma cells we used Western blotting against activating phosphorylation sites on proteins in the RAF-MEK-ERK, AKT and JAK/STAT pathways before and after treatment of cell lines to CX-6258. We observed no significant differences in A375-S and A375-RMR at drug concentrations as high as 1 μM (Figure 2H). In a complementary experiment, we over-expressed wild-type or kinase-dead PIM kinases (PIM-1-KD, PIM-2-KD, PIM-3-KD) (Figure 2I) in A375-S cell lines and observed no change in proliferation rates as compared to EGFP-transduced control cells (p=0.56 and p=0.47, one-way ANOVA) (Figure 2J and K). These data suggest that neither PIM kinases and MYLK4, nor components of signal transduction pathways required for the growth of melanoma cells are responsible for the anti-proliferative effects of CX-6258. We hypothesize that these effects are mediated by inhibition of HASPIN.
The only well-described function of HASPIN is phosphorylation of Histone 3 (H3) on threonine 3 (H3pT3), which promotes binding of the chromosomal passenger complex (CPC) and regulates progression through mitosis(22). Treatment of melanoma cells lines with CX-6258 reduced H3pT3 in a dose dependent fashion (EC50 ~ 150 nM) (Figure 2L and Supplementary Figure 2A). Additionally, transfection of A375-S with previously validated siRNA against HASPIN (Supplementary Figure 2B) resulted in significant reduction in cell numbers as compared to a non-targeting siRNA (−63.86%, p<0.0001, t test) (Figure 2M). Together, these results suggest that HASPIN is a key cellular target of CX-6258 and responsible for its anti-tumor activity, while preserving viability of human TILs and neurons.
On-target inhibition of HASPIN results in reduced proliferation and increased formation of micronuclei
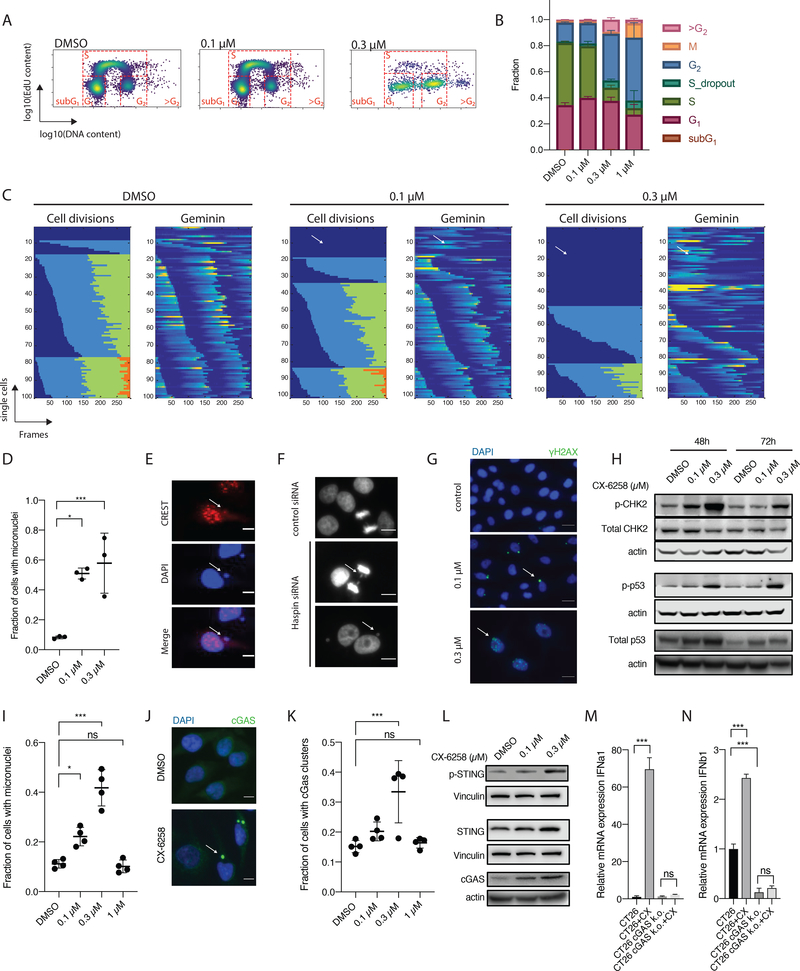
To study the effects of CX-6258 on cell division we performed EdU incorporation assays and found that CX-6258 caused dose-dependent arrest in G2/M in A375 and UACC62 cells (Figure 3A and B, and Supplementary Figure 3A). At higher drug doses, we identified cells with DNA content >4N. This has previously been reported in cells treated with Aurora Kinase inhibitors further supporting CX-6258 acting at the level of CPC recruitment and function(23). These observations were also true in the murine cancer cell line CT26 which was used for the syngeneic mouse studies described below, although we observed a larger number of cells with a DNA content >4N and reduced GRmax compared to human melanoma cell lines (Supplementary Figure 3B and C).
Figure 3.
(A) EdU assay of A375S cells treated with DMSO or CX-6258 as indicated. x axis indicates DNA content (log10), y axis indicates EdU incorporation (log10). In each panel, the different stages of the cell cycle are highlighted as they project into the space of this graph. (B) Fraction of cells (y axis) shown in (A) in each cell cycle stage. Error bars, mean ± SD. (C) Live-cell imaging of A375-S cells treated with DMSO or CX-6258 at indicated doses. Each row represents a single-cell track over time (x axis in frames, images taken every 10 minutes). For each condition, two plots are shown. The right plot indicates cell divisions, where changes in color (i.e from blue to green to orange) indicates a cell division. x axis indicates the chronologically collected number of frames. To the left, intensity in Geminin reporter (mCherry), where accumulation of Geminin (increasing intensity indicated by red color) indicates progression towards a cell division. (D) Fraction of micronuclei containing A375-S (y axis) after 48h treatment with CX-6258. at indicated doses (x axis). (Error bars, mean ± SD. DMSO vs. 0.1 μM, adjusted p-value=0.01, DMSO vs. 0.3 μM, adjusted p-value=0.005, ANOVA). (E) Merged image if immunofluorescence staining of CREST (red) and DAPI (blue). The white arrow indicates a micronucleus which contains chromosomal fragments (as indicated by co-staining of DNA and CREST). (F) Representative image of A375-S cells treated with either a non-targeting siRNA (negative control) (top), or a siRNA against Haspin kinase and imaged over time with chromosome bridges (middle panel) and resulting daughter cells with peri-centric micronuclei (right). (G) Merged IF staining of A375-S staining for γ-H2AX (green) or DAPI (blue) after treatment with DMSO or CX-6258 at indicated doses. At 100 nM, there is γ-H2AX staining is limited to micronuclei (white arrow), at 300 nM there shows higher frequency of double-stranded DNA breaks indicated by nuclear γ-H2AX staining (white arrow). (H) Western blot of total and pCHK2, total and pP53, and actin loading controls in A375-S cells treated for 48 or 72 hours with DMSO or CX-6258 at indicated doses. There is increasing abundance of pCHK2, total and pP53, indicating activation of the DNA damage response pathway in response to CX-6258 treatment. (I) Fraction of micronuclei formation in cGAS proficient UACC62-S (Error bars, mean ± SD. p-value<0.001, ANOVA). (J) Merged IF for cGAS (green) and nuclei (DAPI, blue) in UACC62-S following treatment with DMSO vs. CX-6258 (100 nM). (K) Fraction of cGAS-positive micronuclei across all imaged UACC62-S cells (y axis) following treatment with CX-6258 at indicated doses in x axis or DMSO. (Error bars, mean ± SD. p-value=0.002, ANOVA). (L) Western blot of total and phosphorylated STING (p-STING), cGAS and loading controls (actin and vinculin). (M) mRNA expression of IFNα1 and (N) and IFNβ1 (y axis) in parental CT26 cell lines or corresponding CT26 cGAS-KO (y axis) following treatment with CX-6258 or DMSO. (Error bars, mean ± SD. p-value<0.001, statistical test, ANOVA)
To study mitotic progression, parental A375 cells were transduced with a dual-reporter system (geminin-mCherry and H2B-YFP) followed by live-cell fluorescence microscopy(19). Geminin accumulates during S/G2 and M phases of the cell cycle and is rapidly degraded by the Anaphase Promoting Complex (APC) at the metaphase to anaphase transition; the levels of geminin therefore identify cell cycle stages. Expression of H2B-YFP reveals chromatin morphology and assists with cell tracking. We tracked a total of 307 cells in three conditions for 48 hours (DMSO, n=100; 100 nM CX-6258, n=102; 300 nM CX-6258, n=105). In DMSO treated cells, cells divided (on average) three times (Figure 3C). Treatment with CX-6258 reduced the number of cell divisions in a dose-dependent manner (Figure 3C and Supplementary Figure 3D). A subset of cells failed to undergo chromosome condensation, segregation and cytokinesis but still progressed through the cell cycle, as shown by changes in geminin intensity (i.e. cell #12 in the 0.1 μM treatment group and cell #20 in the 0.3 μM treatment group) (Figure 3C). Cells that progressed through mitosis exhibited mitotic mis-segregation as scored by chromosomal bridges during anaphase and formation of micronuclei in daughter cells (Supplementary Figure 3E–G, Supplementary Video 1 and 2). Treatment with either 0.1 μM or 0.3 μM CX-6258 led to a significant accumulation of cells with post-mitotic micronuclei after 48 h (8.274 % vs. 50.9% for DMSO vs. 0.1 μM, adjusted p value 0.01; 8.274 % vs. 57.8% for DMSO vs. 0.3 μM, p value 0.005; one-way ANOVA; Figure 3D). In CX-6258-treated cells micronuclei co-stained with CREST, a marker for centromeres, suggesting that micronuclei were composed of chromosomes fragmented by abnormal mitotic segregation and supporting a function for HASPIN in mitosis (Figure 3E). To investigate the acute loss of HASPIN on mitotic progression we treated A375-S with previously validated siRNA against HASPIN (Supplementary Figure 2B). This resulted in lagging chromosomes, formation of micronuclei and reduction in proliferation (Figure 3F and 2M), phenocopying the effects of CX-6258. In contrast, neither reduced proliferation nor increased formation of micronuclei were observed in cells with PIM1/2 or MYLK4 CRISPR-Cas9 KO. These data support that on-target inhibition of HASPIN by CX-6258 leads to mitotic errors, resulting in the generation of micronuclei.
Exposure of parental A375 cells to CX-6258 resulted in significant increases in phosphorylated H2A.XS139 (γ-H2AX) foci (DMSO vs. 0.1μM, 0.31 vs 1.09 average number of foci per nucleus, adjusted p value = 0.04; DMSO vs. 0.3 μM, 0.31 vs. 3.94, adjusted p value<0.001, one-way ANOVA). γ-H2AX foci are a marker of double-stranded DNA breaks. At 0.1 μM CX-6258 γ-H2AX staining was predominantly restricted to micronuclei, consistent with their origin in fragmented chromosomes, but at higher doses (0.3 μM) the drug induced multifocal γ-H2AX staining in the nucleus indicative of double-stranded DNA breaks throughout the genome (Figure 3G and Supplementary Figure 3H and I). This triggered the DNA damage response (DDR), as shown by increased phosphorylation of CHEK2 and increased abundance and phosphorylation of p53 (Figure 3H). We conclude that inhibition of HASPIN results in DNA damage and the formation of micronuclei that are prone to rupture.
HASPIN inhibition induces type I interferon response in a cGAS-dependent fashion and reduced tumor growth in vivo
Formation and rupture of micronuclei has previously been shown to trigger the cytosolic DNA-sensor Cyclic GMP-AMP synthase (cGAS)(24). UACC62 cells are cGAS-proficient (Supplementary Figure 3J) and we found that exposure of these cells to CX-6258 at concentrations that still allowed cell division (~0.3 x GRmax; 0.3 μM) triggered micronuclei formation (DMSO vs. 0.3 μM, adjusted p value <0.0001, ANOVA) and resulted in recruitment of cGAS specifically to micronuclei (Figure 3I–K, Supplementary Figure 3K). At drug concentrations high enough to fully block cell division (1 x GRmax=1 μM) the frequency of micronuclei formation was lower and cGAS was not recruited (Figure 3K). Additionally, we observed increased levels and phosphorylation of STING (Stimulator of Interferon Genes), which is triggered by the cGAS product cGAMP, and induction of cGAS itself, consistent with a previously described positive feedback loop (Figure 3L)(25).
cGAS-STING pathway activation results in expression of anti-viral immunity via type I interferons (IFNα and IFNβ) and has potential benefits for anti-tumor immunity(26). To test for induction of a type I-IFN response by CX-6258, we used CT26 murine cells, which are cGAS proficient and can be used to generate tumors in nude and immunocompetent mice. RT-PCR of CT26 cells exposed to CX-6258 at 0.1 μM for five days revealed strong induction of mRNA for both IFNα1 (>60-fold, adjusted p <0.001, ANOVA) and IFNβ1 (2.4-fold, adjusted p value<0.001, ANOVA) (Figure 3M–N). To confirm that cGAS was necessary for IFN induction, we used CRISPR-Cas9 to knockout cGAS in CT26 cells (Supplementary Figure 3L) and observed an ~5-fold reduction in basal IFNβ1 levels (adjusted p value <0.001, ANOVA) with no significant induction of IFNα and IFNβ by CX-6258 (Figure 3M–N). Thus, CX-6258 triggers a type I IFN response in a cGAS-dependent manner.
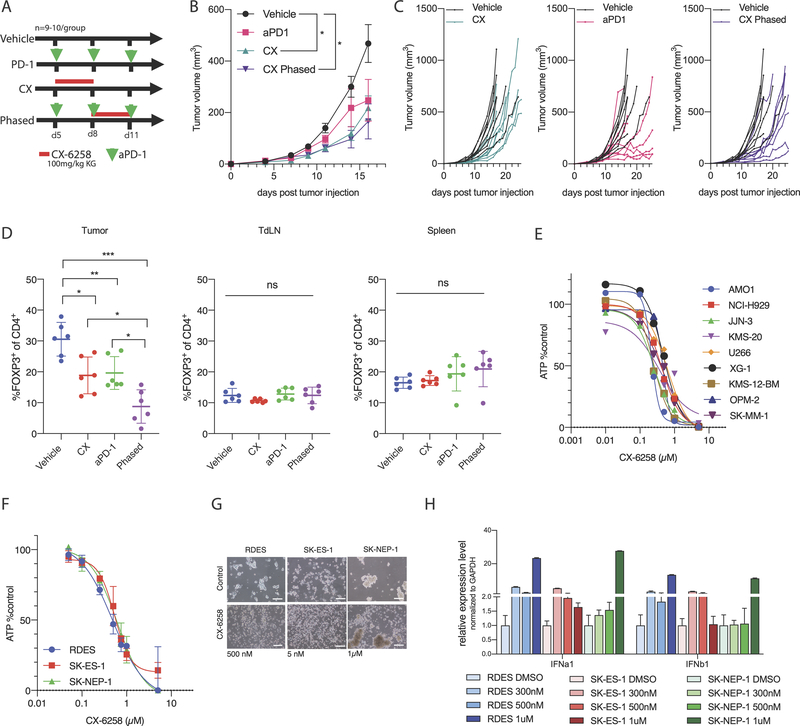
To study the effects of CX-6258 in vivo, nude mice were engrafted with CT26 cells and treated with high and low doses of CX-6258 (10 and 100 mg/kg) for 5 days. Rates of tumor growth were variable (n=5/group), but tumor volumes at day 14 were not significantly different in CX-6258-treated mice and vehicle-only controls (Supplementary Figure 4A and B). The difference in vivo efficacy of CX-6258 in nude mice bearing A375 and CT26 tumors may reflect the fact that the drug is cytoxic in vitro in A375 but not CT26 cells (Figure 1C and Supplementary Figure 3C). To see if adaptive immunity might enhance the effects of CX-6258, CT26 cells were engrafted in immune-competent BALB/c animals and cohorts of 8 animals were exposed to CX-6258, anti-PD-1 antibody, or a phased combination of CX-6258 plus anti-PD-1 (see study design in Figure 4A). Animals treated with high dose CX-6258 alone exhibited a significant reduction in tumor growth as compared to vehicle-only controls (adjusted p value= 0.02, 2-way ANOVA); PD-1 alone did not reach statistical significance (adjusted p value=0.36). Notably, the response to anti-PD-1 was variable and occurred in a delayed fashion. Phased combination of anti-PD-1 and CX-6258 led to a less variable and significant delay in tumor outgrowth (adjusted p value < 0.002, 2-way ANOVA) (Figure 4B and C). The body weight of all treatment groups was higher compared to vehicle, and this was due to tumor outgrowth and associated effects in the vehicle control (Supplementary Figure 4C). Thus, the anti-tumor activity of CX-6258 is substantially enhanced by the presence of an adaptive immune system, being substantially greater in immune-competent than in nude mice. All treatment modalities significantly increased median survival compared to vehicle (Supplementary Figure 4D) and phased combination delayed outgrowth compared to anti-PD-1 but did not provide an additional benefit in median survival over anti-PD-1 alone (Vehicle vs. anti-PD-1, p=0.02; Vehicle vs. CX, p=0.04; Vehicle vs. phased, p=0.002).
Figure 4.
(A) Study design for in vivo in BALB/c mice implanted with CT26 tumors. Green arrow heads indicate days after tumor injection (D5, D8 and D11) animals were treated with intraperitoneally (IP) administered anti-PD-1 antibody; the red bar indicates days animals were treated with CX-6258 by oral gavage. Top group were treated with vehicle controls, second with anti-PD-1 alone, third with CX-6258 alone and the last group was treated with a phased combination of anti-PD-1 and CX-6258. N=8 animals/group. (B) Growth curves of animals treated per design in (A), x axis indicating days since tumor injection and y axis indicating tumor volume in mm3.( Error bars, mean ± SEM. Vehicle vs. CX, adjusted p-value=0.02; vehicle vs. aPD-1, adjusted p-value=0.36; vehicle vs. phased, adjusted p-value=0.002, 2way ANOVA), (C) Growth curves of individual animals treated per design in Figure 4A, x axis indicating days since tumor injection and y axis indicating tumor volume in mm3. (D) Flow-cytometry analysis of lymphocytes of animals implanted with CT26 tumors and treated as per treatment scheme in Figure S4E, x axis indicating different treatment groups, and y axis indicating % of FOXP3 positive cells of all CD4+ cells in tumor, tumor-draining lymph nodes (tdLN) and spleens. Analysis of N=6 animals/group. Error bars, mean ± SD. p-value<0.001, ANOVA). (E) Multiple myeloma cell lines (names indicated on the right) treated with CX-6258, x axis indicating doses used in μM, y axis indicating % of ATP levels per CellTiterGlo assay compared to DMSO treated control. (F) Ewing sarcoma cell lines (names indicated in bottom left) treated with CX-6258, x axis indicating doses used in μM, y axis indicating % of ATP activity per CellTiterGlo assay compared to DMSO treated control. (G) Impact of CX-6258 treatment (right) vs. DMSO (left) on spheroid formation of Ewing sarcoma cell lines shows variable responses. Scale bar represents 200 μm. (H) Relative expression of IFNa1 and IFNb1 mRNA in Ewing sarcoma cell lines treated with increasing doses of CX-6258 (indicated by color gradient as defined at the bottom of the graph), y axis relative gene expression normalized to DMSO control in each cell line.
We hypothesized that response to CX-6258 (either alone or in phased combination with anti-PD-1 therapy) could be due to modulation of the tumor microenvironment (TME). To test this hypothesis, we treated animals with a similar treatment design (Supplementary Figure 4E) but with a lower dose of CX-6258 (10 mg/kg) resulting in similar tumor volumes in all groups on day 15 after tumor implantation (Supplementary Figure 4F). Tumors, tumor-draining lymph nodes and spleens were then harvested and subjected to analysis by flow-cytometry (n=5–6 animals per group) (Supplementary Figure 4G). The rate of proliferating immune cells as assessed by Ki67 staining was not different between treatment groups (Supplementary Figure 4H). Compared to vehicle-only controls, CX-6258-alone increased infiltration of CD3+ T lymphocytes into the TME (31% vs. 43%, adjusted p=0.04, ANOVA) (Supplementary Figure 4I). The most significant difference between treated and untreated animals was in the abundance of regulatory CD4+ T cells (Tregs). Significant reduction in Tregs was observed in tumors treated with CX-6258 (30.57% vs. 18.8% of CD4+, adjusted p value = 0.008, ANOVA) or anti-PD-1 therapy (30.57% vs. 19.63% of CD4+, adjusted p value = 0.01, ANOVA) as compared to vehicle-only controls and this reduction was most pronounced in animals receiving the phased combination (30.57% vs. 10.97% of CD4+, adjusted p value < 0.001, ANOVA) (Figure 4D). In contrast, there was no significant change in Tregs in tumor draining lymph nodes (tdLNs; p=0.29, ANOVA) or spleens (p=0.27, ANOVA) harvested from the same animals, indicating a specific effect in the TME (Supplementary Figure 4I). We also observed increased infiltration of IFNγ-producing CD8+ T and NK cells (Supplementary Figure 4J–K). These data suggest that CX-6258 modulates the immune environment in tumors to favour a more consistent response to immune checkpoint blockade. HASPIN inhibition may therefore promote anti-tumor activity by directly inhibiting tumor growth and also improving anti-tumor immunity.
HASPIN inhibition may be effective in the treatment of other human cancer contexts
To investigate the therapeutic potential for HASPIN inhibition in other human cancers, we performed a pan-cancer analysis of gene expression in The Cancer Genome Atlas. We found that HASPIN was strongly overexpressed in a wide range of cancer types (Supplementary Figure 4L). Next we queried the Broad Institute Dependency Map(27) for cancer cell lines dependent on HASPIN expression. Multiple myeloma (MM) cell lines (DEMETER2 dependency score −0.1, p<0.0001) and Ewing sarcoma (ES) (CERES dependency score −0.8, p<0.0001) were predicted to be more sensitive to inhibition of HASPIN as compared to other lineages. We therefore tested the activity of CX-6258 in nine MM cell lines (AMO1, NCI-H929, JJN-3, KMS-20, U266, XG-1, KMS-12-BM, OPM-2 and SKMM-1) and three ES cell lines (RDES, SKES1 and SKNEP1). CX-6258 inhibited cell growth in all MM cell lines tested with EC50 < 1 μM (Figure 4E). In ES cell lines, CX-6258 was also active at EC50 < 1 μM (Figure 4F). Additionally, CX-6258 inhibited formation of ES spheroids; in this assay, cells are grown on a low-adherent surface to induce formation of sarcoma-spheres. In SKES1, CX-6258 prevented the formation of spheroids at 5 nM; in RDES and SKNEP1 there was a significant reduction of spheroid formation at 500 nM and 1μM, respectively (Figure 4G). Treatment of ES cell lines with CX-6258 also led to induction of IFNα1 and IFNβ1 across all lines (Figure 4H). Overall, these results indicate that CX-6258 may be active in multiple human cancer cell lines, while exhibiting modest toxicity against normal tissues.
DISCUSSION
While most patients with advanced/metastatic melanoma harbouring BRAFV600E/K develop resistance to treatment with RAF/MEK inhibitors (RMI), they also become less responsive to subsequent therapies, including ICI(7–10). We show that the small-molecule CX-6258 blocks cell proliferation and induces cell death of both RMI-sensitive and RMI-resistant (RMR) BRAF-mutated melanoma lines, most likely by inhibiting HASPIN kinase, which promotes errors in mitotic chromosome segregation. Primary human TILs and neurons differentiated in vitro are substantially less sensitive to CX-6258 than tumor cells, suggesting a potential therapeutic window for future clinical application. CX-6258 has in vivo activity in immunocompetent mice and results in significant modulation of the tumor immune environment, including reduction in Tregs and increase in cytotoxic CD8+ T cells, thereby contributing to a more consistent response to immunotherapy.
CX-6258 is annotated as an inhibitor of the PIM kinase family(15). However, using unbiased kinase inhibitor profiling, we identified MYLK4 and HASPIN as additional high affinity targets of CX-6258. Overexpression of PIMs did not alter the proliferation rates of cell lines in which CX-6258 was active nor did knockdown of PIM1, PIM2 or MYLK4 alter sensitivity to CX-6258. BAD is an established downstream target of PIMs but CX-6258 did not measurably reduce pBAD levels at the doses tested, showing that CX-6258 is active on cells under conditions in which PIM signaling is still active. In contrast, knockdown of HASPIN phenocopied CX-6258 with respect to cell proliferation; HASPIN knockdown also resulted in mitotic mis-segregation and formation of micronuclei and increased the number of double-stranded DNA breaks. CX-6258 also caused dose-dependent reductions in Histone H3 phosphorylation at Threonine 3, the only known phosphorylation site dependent on HASPIN. Our data strongly suggests that HASPIN is a biologically significant target of CX-6258 in cancer cells, although they do not rule out a secondary role for PIMs or MYLK4.
Some of the other compounds we tested in this study, such as SGI1776, have a similar affinity for HASPIN in biochemical assays compared to CX-6258, but did not demonstrate significant anti-tumor activity in both classical viability assays and following growth rate correction. This may be due to several factors, including the higher molecular lipophilicity potential CX-6258, retention within the cell (as indicated by yellow discoloration of cells at high concentrations representing the endogenous fluorescence signal of CX-6258), and possibly by inhibiting its own transport outside of the cell via ABC transporters, as recently reported(28).
Mitotic errors resulting from CX-6258 treatment result in formation of rupture-prone micronuclei. These micronuclei stain positive with CREST antibodies suggesting that they arise through errors in mitotic chromosome segregation and not just DSB formation. This is consistent with a role for HASPIN in mitosis. MCF10A breast cancer cell lines exposed to ionizing radiation for 3 to 6 days also develop micronuclei as a consequence of double-stranded DNA-breaks(29). Formation of micronuclei requires progression through mitosis and is not observed in cells arrested in G2/M. Consistent with this result, micronuclei generated in response to HASPIN inhibition with CX-6258 required progression through mitosis, and while non-divisions may result in accumulation in DSBs, they were not the source for MN. This suggests that MN may evolve from different types of DNA damage, however, appear to be a result of mitotic errors during HASPIN inhibition.
Rupture of micronuclei release DNA to the cytosol which in turn triggers the cytosolic DNA-sensor cGAS(26). We find that micronuclei recruit cGAS, as recently reported(30). While binding of cGAS to cytosolic DNA is weaker than to nuclear DNA, it has recently been reported that signaling via the cGAS-STING pathway is more potent when triggered by cytosolic DNA(31). Consistent with this finding, we see increased phosphorylation of STING, stabilization of STING and induction of cGAS itself in response to cytosolic DNA. Chromosomal instability (CIN) resulting from errors in mitotic segregation has been associated with increased metastatic potential through tonic STING activation(32) resulting in an immunosuppressive tumor environment(33). However, acute induction of CIN as observed with CX-6258, yields a type I interferon response and are less likely to be propagated(33). In line with this, we show that HASPIN inhibition results in a cGAS-dependent induction of type I interferon by tumor cells. This cascade promotes several changes in the tumor immune environment, including a reduction of immunosuppressive Tregs and increase in IFNγ producing T cells, both of which are known to enhance response to ICI. Reduction of Tregs mediated by inhibitors of cell cycle or mitosis has been observed in the context of CDK4/6 inhibitors, and may indicate differential yet poorly defined sensitivity of Tregs to anti-proliferative compounds(34,35,11), including HASPIN inhibitors.
Several inhibitors of mitotic kinases have been tested in clinical trials. Properly selected genetic subsets of tumors, such as RB1-deficient small cell lung cancer, exhibit sensitivity to Aurora kinase B inhibitors(36), suggesting that anti-mitotic agents could be beneficial in the appropriate clinical context. However, Aurora kinases are involved in multiple steps in mitosis and exhibit non-canonical activities, which may in part explain the broad side effect profile in patients(37). Unlike other drug targets involved in mitosis, HASPIN has only one well-described function and our study further supports this notion.
HASPIN is broadly, and more highly expressed in various cancer types compared to the corresponding non-malignant tissue, both may make its inhibition relatively tumor-specific. In addition to the effects observed in melanoma cell lines, we find that HASPIN inhibition may represent a therapeutic strategy in other cancers, including myeloma and Ewing sarcoma. These findings support a role for HASPIN as a target for direct inhibition of tumor growth in addition to the potential beneficial activity in combination with immune checkpoint inhibitors.
While our study provides evidence that inhibition of HASPINs’ role in mitosis is the primary mechanism to promote anti-proliferative activity and enhanced immunogenicity, it is possible that other, currently unknown downstream targets of HASPIN could contribute to these effects. Furthermore, inhibition of CX-6258 targets, including PIMs and HASPIN, in different cell types could in concert explain anti-tumor activity. For instance, while HASPIN is the key target in tumor cells, PIM2 was recently identified as negative regulator of T cell function(38), and its inhibition may in part explain enhanced production of IFNγ in CD8+ T cells, as shown here. This provides rationale for developing tumor-selective inhibitors of HASPIN with PIM2 inhibitory function in T cells.
In summary, our study characterizes a potent inhibitor of HASPIN, and identifies this mitotic kinase as an interesting target for drug therapy, because it results in both direct anti-tumor activity and modulates the tumor microenvironment which may enhance response to immunotherapies.
Supplementary Material
SIGNIFICANCE.
Haspin Kinase inhibition by CX-6258 is a novel and potent strategy for RAF/MEK-inhibitor resistant melanoma and potentially other tumor types. HASPIN inhibition has direct anti-tumor activity and induces a favorable immune-microenvironment.
ACKNOWLEDGEMENTS
We thank Dr. Nikhil Munshi for discussions and support of this work. This work was supported by NCI K08CA222663 (to B.I.), NIH U54CA225088 (to P.K.S.), Burroughs Wellcome Fund Career Award for Medical Scientists (to B.I.), Ludwig Center for Cancer Research (to K.W., P.K.S. and B.I.), Barr Award for Cancer Research (to B.I.), and Society for Immunotherapy of Cancer (to B.I.), and NCI R01CA238039 (to K.W.), and NCI P01155258 (PI: Nikhil Munshi, Dana-Farber Cancer Institute). S.K. was supported by an AACR-AstraZeneca Cancer Research Fellowship. J.C.M. and B.I. filed a provisional patent on Haspin kinase as target for cancer therapy. B.I. reports personal fees from Merck & Co. D.S. reports personal fees from Amgen, personal fees from Boehringer Ingelheim, personal fees from Leo Pharma, personal fees and other from Roche, personal fees and other from Novartis, personal fees from Incyte, personal fees and other from Regeneron, personal fees from 4SC, personal fees from AstraZeneca, personal fees and other from BMS, personal fees from Pierre Fabre, personal fees and other from Merck-EMD, personal fees from Pfizer, personal fees and other from Philiogen, personal fees from Array, personal fees and other from MSD, none of which are related to the work presented here.
Footnotes
Conflict of interest statement:
J.C.M and B.I. filed for a provisional patent on the use of Haspin Kinase inhibitors for cancer therapy. B.I. reports personal fees from Merck & Co. D.S. reports personal fees from Amgen, personal fees from Boehringer Ingelheim, personal fees from Leo Pharma, personal fees and other from Roche, personal fees and other from Novartis, personal fees from Incyte, personal fees and other from Regeneron, personal fees from 4SC, personal fees from AstraZeneca, personal fees and other from BMS, personal fees from Pierre Fabre, personal fees and other from Merck-EMD, personal fees from Pfizer, personal fees and other from Philiogen, personal fees from Array, personal fees and other from MSD, none of which are related to the work presented here. All other authors have no conflict of interest related to the work published in this manuscript.
REFERENCES
- 1.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat J-P, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB, et al. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: targeted strategies for melanoma. Nat Rev Cancer. 2012;12:349–61. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–82. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, Lawrence DP, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695–701. [DOI] [PubMed] [Google Scholar]
- 8.Amini-Adle M, Khanafer N, Le-Bouar M, Duru G, Dalle S, Thomas L. Ineffective anti PD-1 therapy after BRAF inhibitor failure in advanced melanoma. BMC Cancer. 2018;18:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su M-J, Melms JC, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell. 2018;175:984–997.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruscetti M, Leibold J, Bott MJ, Fennell M, Kulick A, Salgado NR, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362:1416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantelidou C, Sonzogni O, De Oliveria Taveira M, Mehta AK, Kothari A, Wang D, et al. PARP Inhibitor Efficacy Depends on CD8+ T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019;9:722–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falchook GS, Bastida CC, Kurzrock R. Aurora Kinase Inhibitors in Oncology Clinical Trials: Current State of the Progress. Semin Oncol. 2015;42:832–48. [DOI] [PubMed] [Google Scholar]
- 15.Haddach M, Michaux J, Schwaebe MK, Pierre F, O’Brien SE, Borsan C, et al. Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor. ACS Med Chem Lett. 2012;3:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eswaran J, Patnaik D, Filippakopoulos P, Wang F, Stein RL, Murray JW, et al. Structure and functional characterization of the atypical human kinase haspin. Proc Natl Acad Sci U S A. 2009;106:20198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafner M, Niepel M, Chung M, Sorger PK. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat Methods. 2016;13:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallahi-Sichani M, Becker V, Izar B, Baker GJ, Lin J-R, Boswell SA, et al. Adaptive resistance of melanoma cells to RAF inhibition via reversible induction of a slowly dividing de-differentiated state. Mol Syst Biol. 2017;13:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sucker A, Zhao F, Pieper N, Heeke C, Maltaner R, Stadtler N, et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat Commun. 2017;8:15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H, Frederick DT, Levesque MP, Cooper ZA, Feng Y, Krepler C, et al. Downregulation of the Ubiquitin Ligase RNF125 Underlies Resistance of Melanoma Cells to BRAF Inhibitors via JAK1 Deregulation. Cell Rep. 2015;11:1458–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai J, Higgins JMG. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle Georget Tex. 2005;4:665–8. [DOI] [PubMed] [Google Scholar]
- 23.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–7. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- 25.Ma F, Li B, Liu S, Iyer SS, Yu Y, Wu A, et al. Positive Feedback Regulation of Type I IFN Production by the IFN-Inducible DNA Sensor cGAS. J Immunol. 2015;194:1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ablasser A, Chen ZJ. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363. [DOI] [PubMed] [Google Scholar]
- 27.Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, et al. Defining a Cancer Dependency Map. Cell. 2017;170:564–576.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson BG, Tan KW, Osa-Andrews B, Iram SH. High-content screening of clinically tested anticancer drugs identifies novel inhibitors of human MRP1 (ABCC1). Pharmacol Res. 2017;119:313–26. [DOI] [PubMed] [Google Scholar]
- 29.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L, et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature. 2018;563:131. [DOI] [PubMed] [Google Scholar]
- 31.Zierhut C, Yamaguchi N, Paredes M, Luo J-D, Carroll T, Funabiki H. The Cytoplasmic DNA Sensor cGAS Promotes Mitotic Cell Death. Cell. 2019;178:302–315.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakhoum SF, Ngo B, Laughney AM, Cavallo J-A, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakhoum SF, Cantley LC. The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell. 2018;174:1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8:216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oser MG, Fonseca R, Chakraborty AA, Brough R, Spektor A, Jennings RB, et al. Cells Lacking the RB1 Tumor Suppressor Gene are Hyperdependent on Aurora B Kinase for Survival. Cancer Discov. 2018;CD-18–0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melichar B, Adenis A, Lockhart AC, Bennouna J, Dees EC, Kayaleh O, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. [DOI] [PubMed] [Google Scholar]
- 38.Daenthanasanmak A, Wu Y, Iamsawat S, Nguyen HD, Bastian D, Zhang M, et al. PIM-2 protein kinase negatively regulates T cell responses in transplantation and tumor immunity. J Clin Invest. 2018;128:2787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.