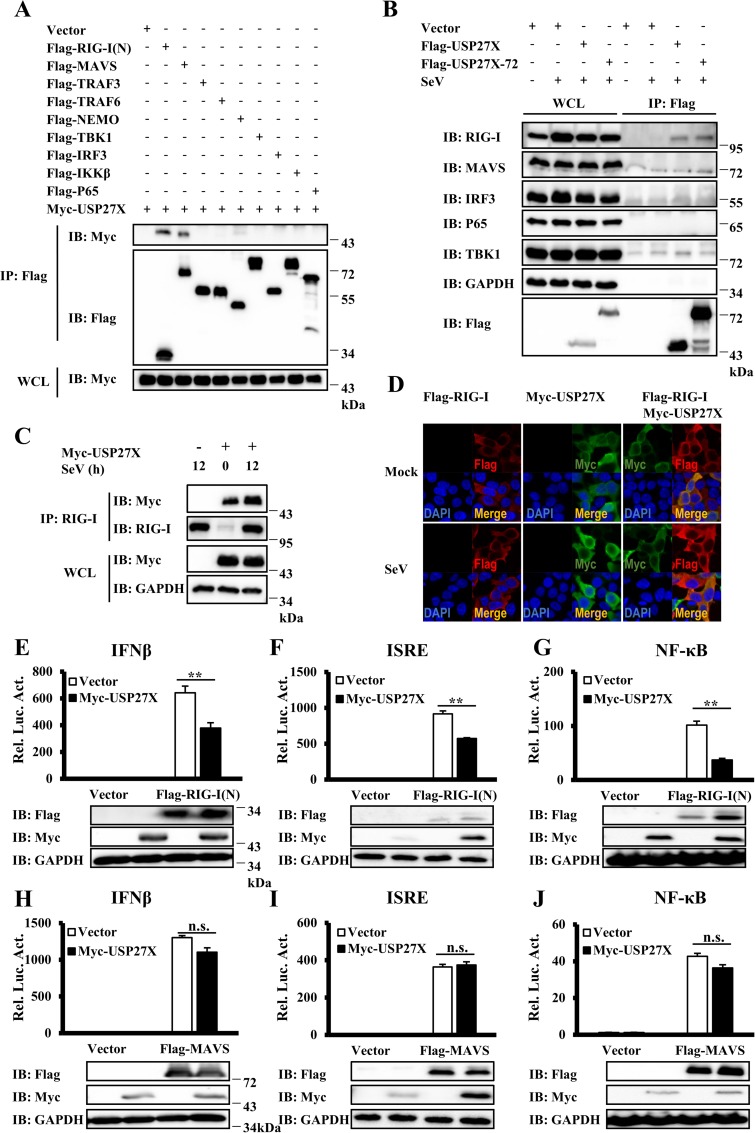
Fig 4. USP27X targets RIG-I to regulate antiviral signaling.
(A) HEK293T cells were transfected with the indicated expression plasmids. Twenty-four hours after transfection, the cells were lysed for Co-IP with anti-Flag agarose beads, followed by immunoblotting. The expression levels of transfected proteins in whole cell lysates (WCL) are shown in the bottom panels. (B) HepG2 cells were transfected with Flag-USP27X, or Flag-USP27X-72 expression vector or empty vector. Twenty-four hours after transfection, the cells were mock-infected or infected with SeV for 18 h. Cell lysates were immunoprecipitated with anti-Flag beads, followed by immunoblotting with the indicated antibodies. (C) HEK293T cells were transfected with Myc-USP27X expression vector or empty vector. Twenty-four hours after transfection, the cells were mock-infected or infected with SeV for 12 h. Cell lysates were immunoprecipitated with anti-RIG-I antibody, followed by immunoblotting. (D) HEK293T cells were transfected with the indicated expression plasmids. Twenty-four hours after transfection, cells were mock-infected or infected with SeV (50HA) for 9 h. The cells were fixed, stained with the anti-Flag (red) and anti-Myc (green) antibodies, and observed by confocal microscopy. (E–G) HEK293T cells were co-transfected with the indicated expression plasmids along with luciferase reporter constructs driven by promoters of IFNβ (E), ISRE (F) or NF-κB (G) as well as Renilla as an internal control. Twenty-four hours after transfection, the cells were lysed for luciferase assays (upper panel) and immunoblotting assays (lower panels). (H–J) Similar to (E–G), except that expression plasmids MAVS were used instead of RIG-I(N). The data shown in (E–J) are from one representative experiment of at least three independent experiments (mean ± SD of duplicate experiments). The two-tailed Student’s t-test was used to analyze statistical significance. **P < 0.01; n.s. not significant versus control groups.