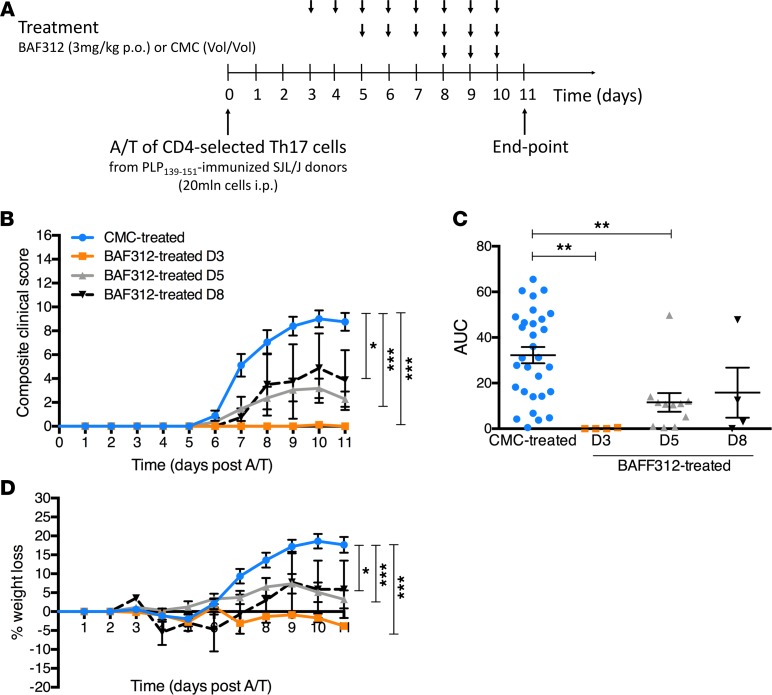
Figure 4. Therapeutic treatment with BAF312 ameliorates clinical outcome in A/T SJL/J EAE mice.
(A) Schematic of the A/T SJL/J EAE protocol integrating the BAF312 or vehicle control (carboxymethylcellulose, CMC) treatment timeline. (B) Composite clinical score (16-point scale) of A/T SJL/J EAE recipient mice treated with CMC (n = 30) or BAF312 over time (from day 3, n = 4; from day 5, n = 12; from day 8, n = 4). (C) Area under the curve (AUC) cumulative disease score for each group. (D) Percentage weight loss of A/T SJL/J EAE recipient mice treated with BAF312 or vehicle control over time. Values are shown as mean ± SEM, and statistical significance was determined by 2-way ANOVA, with *P < 0.05 and ***P < 0.001 in B, or by 1-way ANOVA, with **P < 0.01 in C.