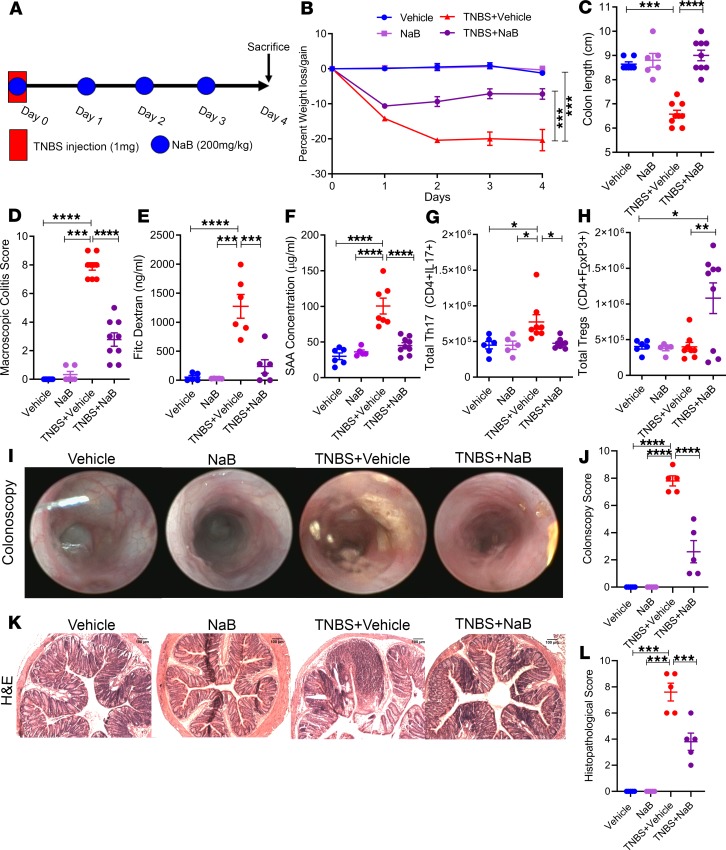
Figure 4. Treatment with NaB resulted in amelioration of TNBS-induced colitis.
(A) TNBS colitis was induced in mice to test the efficacy of treatment with NaB with the following experimental groups: Vehicle (n = 7), NaB (n = 6), TNBS + Vehicle (n = 9), and TNBS + NaB (n = 9), with data combined from 3 independent experiments unless otherwise stated. (B–D) Disease parameters accessed included percent weight loss (B), colon length (C), and macroscopic score (D). (E) FITC-dextran was given to experimental mice by oral gavage on day 3 of the TNBS model, and serum was collected 4 hours later to test gut permeability for Vehicle (n = 5), NaB (n = 5), TNBS + Vehicle (n = 6), and TNBS + NaB (n = 6). Data depicted are combined from 2 independent experiments. (F) On day 3, serum was also collected to determine the levels of circulating SAA for Vehicle (n = 6), NaB (n = 5), TNBS + Vehicle (n = 7), and TNBS + NaB (n = 9). Data are combined from 2 independent experiments. (G and H) Total cell numbers of T cell subsets in the MLN were determined for Th17 (G) and Tregs (H) for Vehicle (n = 6), NaB (n = 5), TNBS + Vehicle (n = 9), and TNBS + I3C (n = 9). (I and J) Representative colonoscopy images (I) taken during day 3 of the TNBS model and scored (J; n = 5). (K and L) Representative H&E stains (K) were scored appropriately (L; n = 5). Scale bars: 100 μm (original magnification, ×100). Images are combined representative samples from 3 independent experiments. Data are shown as mean ± SEM, and significance was determined using 1-way ANOVA and Tukey’s multiple comparisons test; *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001.