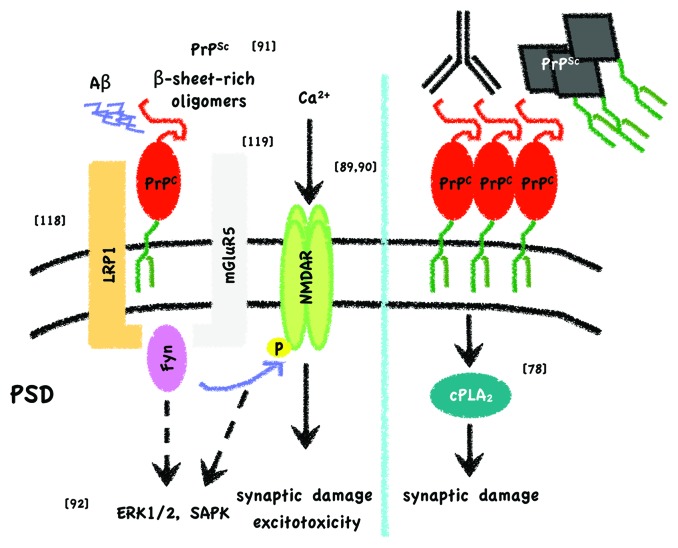
Figure 2. Simplified scheme of toxic signaling through PrPC. On the one hand (left) it has been described that PrPC can bind Aβ oligomers at the post-synaptic density (PSD), either derived from recombinant sources or from brains of Alzheimer disease patients, and elicit toxicity through Fyn activation, NMDAR phosphorylation and altered localization.88,90 Phosphorylation of NMDAR subunits would then lead to dendritic spine loss and excitotoxicty. As long as PrPC is attached to the outer leaflet of the membrane and Fyn to the inner, it has been proposed that a transmembrane protein should act as a scaffold. LRP1118 and mGluR5119 have been suggested as the interacting transmembrane partners after Aβ oligomer binding to PrPC. Resenberger et al. also showed that this signaling cascade can apply for various β-sheet rich conformers.91 In the case of PrPSc binding, apart from Fyn activation, other signaling targets, such as ERK1/2, p38 and JNK, are seen to be activated in neuronal cell lines.92 In addition, it might be hypothesized that β-sheet rich oligomer-associated NMDAR activation per se induces MAP kinase pathways. On the other hand (right) experiments by Bate and Williams demonstrated that after either crosslinking of PrPC with antibodies or by applying PrPSc to cortical primary neurons, there is an increase in membrane cholesterol content that leads to a toxic pathway implicating cPLA2 and arachidonic acid metabolites.78 Reference to original studies is given by the numbers in brackets.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.