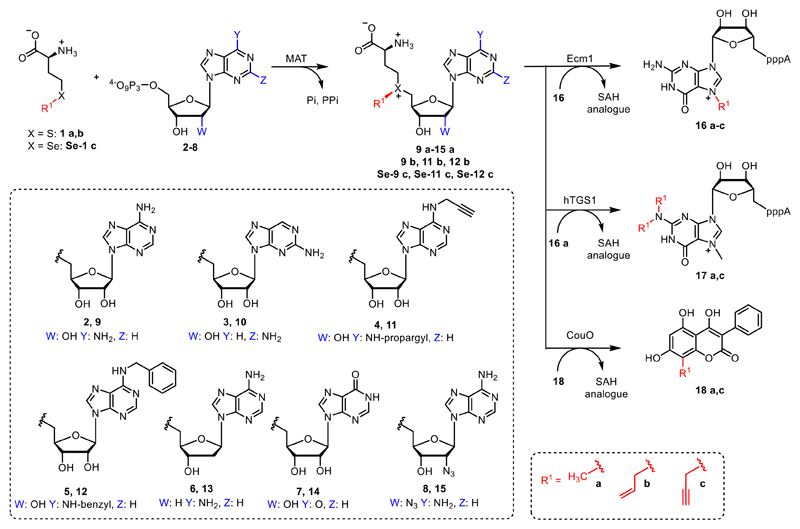
Figure 2.
Methionine (1a) is enzymatically converted by MAT with ATP (2) or different nucleoside triphosphates (3-8) to form AdoMet (9a) or NM-AdoMet analogues (10-15a) with modifications in the nucleoside moiety. These NM-AdoMet analogues are accepted by methyltransferases as shown for Ecm1 in an enzymatic cascade reaction. Furthermore, methionine analogues (1b, Se–1c) and ATP analogues (4, 5) can be converted to AdoMet analogues (11b, Se-11c, 12b and Se-12c) by MAT enzymes. These AdoMet analogues provide selectivity among MTase catalyzed alkylation reactions.