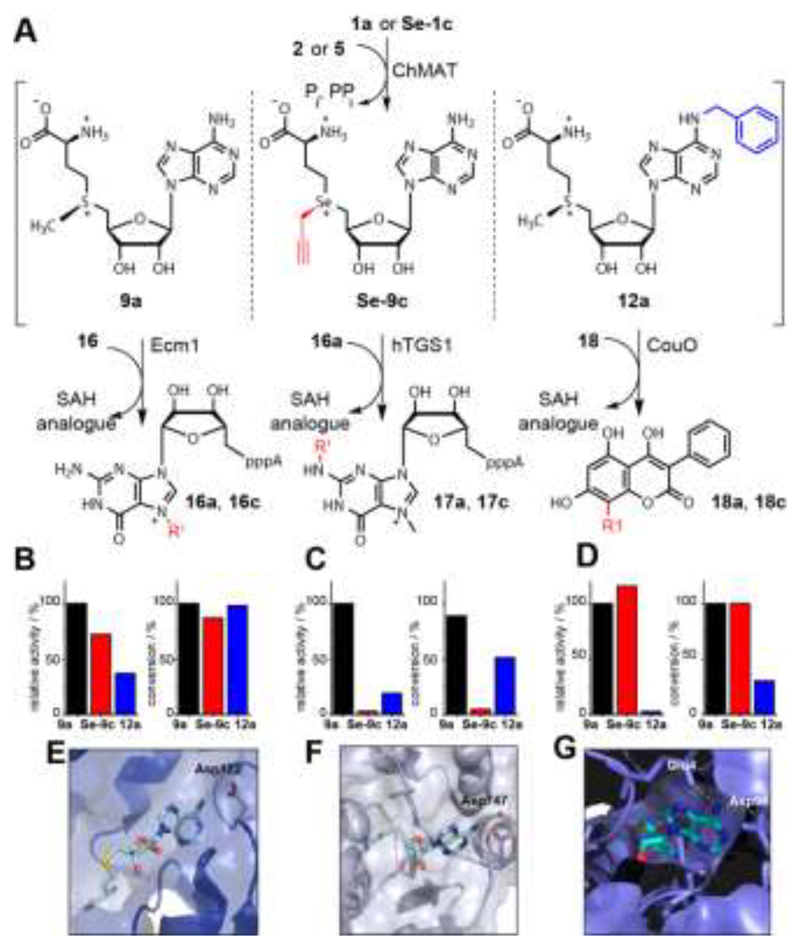
Figure 4.
Differential MTase targeting by AdoMet analogues bearing no modification, a modification at the sulfur/selenium atom or at the nucleoside. A) Reaction scheme. B)-D) Relative activities and maximum conversions for indicated AdoMet analogues for Ecm1 (B), hTGS1 (C) or CouO (D), respectively. Conditions: 6 mM 1a or Se-1c, 2.5 mM 2 or 5, 4 mol% ChMAT (rel. to 2 or 5), 300 μM 16, 16a, or 18, 10 mol% MTase (rel. to 16, 16a, or 18). Reaction mixture is incubated at 23 °C for 30 min (relative activities) or 8h (conversions). E)-G) Crystal structures of Ecm1 (PDB: 1RI1), hTGS1 (PDB: 3GDH) and CouO (PDB: 5M58). For SAH (cyan sticks) polar interactions of the N6 position with the enzyme are highlighted with yellow dashed lines. In E-G) the residues coordinating the N6 position of SAH are labeled.