Abstract
OBJECTIVES
A rise in incidence of sexually transmitted infections (STIs) has been noted in the United States and in the District of Columbia. We aim to describe changes in incident STIs among persons in care for HIV in Washington, DC as well as trends in HIV viral load among those with incident STIs.
METHODS
We conducted a retrospective DC Cohort analysis (n=7810) measuring STI incidence (syphilis, gonorrhea, and chlamydia) as well as in care viral load (ICVL) and percentage with all viral loads less than the limit of detection (%<LLOD) by year (2012–2016) among those with incident STIs.
RESULTS
From 2012–2016, the incidence of STIs increased: chlamydia 2.1 to 3.4 cases/100P-Y (p = 0.0006), gonorrhea 2.1 to 4.0 (p < 0.0001), syphilis 1.7 to 2.6 (p = 0.0042), and any STI episode 5.3 to 8.8 (p<0.0001). STI incidence rates increased for those 18–34 (13.2 to 23.2 cases/100PY, p < 0.0001), cisgender men (6.5 to 11.5, p < 0.0001), non-Hispanic Whites, (8.6 to 16.1, p = 0.0003), and MSM (9.3 to 15.7, p < 0.0001). During 2012–2016, the ICVL among those with incident STIs improved from 108 to 19 copies/ml and %<LLOD from 23.6% to 55.1%. However, even in 2016, younger participants, cis-and transgender females, Non-Hispanic Blacks and Hispanics had higher ICVLs and lower %<LLOD.
CONCLUSIONS
Rates of incident STIs rose among persons in care for HIV in Washington, DC with improved, but not optimal measures of HIV viral suppression. These findings inform focused interventions toward preventing STI transmission and ending the HIV epidemic.
Keywords: HIV, sexually transmitted infection, incidence, chlamydia, gonorrhea, syphilis
INTRODUCTION:
Sexually transmitted infection (STI) incidence has been rising in the United States in recent years, with nationwide surveillance figures showing a 67% rise in cases of gonorrhea from 2013 to 2017, 35% rise in chlamydia, and a near-doubling in cases of syphilis during the same period.1 Factors underlying these rises are posited to include a perception of reduced risk of HIV infection in the age of effective antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP), leading to a decrease in condom use and increase in the transmission of other STIs.2 Incident STIs are a well-known marker of risk for HIV acquisition and transmission.3 Therefore, understanding factors associated with incident STIs among PLWH and measures of viral control over time are necessary not only to contain the spread of STIs, but also to combat the HIV epidemic.
STI trends in Washington, DC largely reflect national trends, with a 35% increase in cases of chlamydia reported to the DC Department of Health (DOH), 56% increase in gonorrhea, and 13% increase in primary and secondary syphilis from 2013 to 2017.4 These figures however, do not describe STI rates among persons living with HIV infection.
The District of Columbia also has one of the highest HIV prevalence rates nationwide, at 1.9% of the general population in 2017.4 Concerted district-wide efforts have succeeded in decreasing the number of new HIV diagnoses in the city from a peak of 1,367 in 2007 to about 350 per year in 2017.4 Sexual transmission, however, particularly among men who have sex with men (MSM), remains a key driver of the HIV epidemic in DC.4 Measures of HIV viral control may help approximate the frequency at which PLWH acquiring an STI are also transmitting HIV infection.
The DC Cohort is a clinic-based, citywide, longitudinal observational cohort that has enrolled over 9,000 people in care for HIV at 15 clinics in Washington, DC since 2011. We have previously described STI incidence of 6.7% among DC Cohort participants during a median observation time of 32.5 months between 2011–2015.5 Additionally, in a multivariate analysis we found that younger age (18–34 years), Hispanic ethnicity, MSM risk, and higher nadir CD4 counts were strongly associated with STI occurrence, whereas the presence of a mental health diagnosis was associated with a lower STI risk.5 We also learned that data reported through cohort sites does not fully capture the full extent of STI occurrence as STI testing and treatment is pursued at locations other than the primary site of HIV care. We therefore conducted a follow-up study linking DC Cohort data to STI surveillance data from the DC DOH for 2012–2016. This analysis aimed to describe trends over time in incident STIs, among people receiving care for HIV in DC in 2012–2016. We also described trends in VLs over time among those with STIs as a means to approximate changes in HIV transmission risk among those participants who have evidence of having sexual risk behavior based on their incident STI.
METHODS:
Study Population and Design:
The DC Cohort study was approved by the George Washington University Institutional Review Board (IRB), and sites with their own IRB also obtained IRB approval to participate. Participants in the DC Cohort consent to have demographic and clinical data electronically and manually abstracted from medical records at the participating sites and entered into a centralized database (Discovere®; Cerner Corporation, Kansas City, MO). Details of the DC Cohort design and data collection have been described in greater detail previously.6,7 At the time of analysis, there were 15 participating sites (9 hospital-based clinics, 5 community-based clinics, and one non-public managed care organization). All sites are experienced HIV care providers and among the adult clinics, provide care to 1000 to 3000 persons with HIV for each of five sites, and the remaining sites each care for 200–1000 persons. Although all sites provide STI screening, evaluation and treatment, the extent of STI screening is not standardized across institutions and can vary within sites across HIV treatment clinicians. One clinic does not participate in the linkage and therefore was not included in this analysis. Data from the DC Cohort was linked to STI surveillance data from the DC Department of Health to capture incident STI diagnoses reported at sites other than the usual location of HIV care.
Eligible participants enrolled in the DC Cohort from 2011 to 2016. Participant follow-up time for this analysis included time from enrollment to the end of 2016, or until the first of these occurred: death, withdrawal from the DC Cohort, or loss to follow-up. Socio-demographic characteristics were collected at study enrollment and updated annually by trained research assistants. Additionally, age was determined at the start of each calendar year of follow-up or at the time of consent, if consented within the calendar year. We examined the incidence of confirmed cases of chlamydia, gonorrhea, and syphilis, as well as the HIV RNA viral load (copies/ml) (VL) in participants.
Participants newly diagnosed with chlamydia, gonorrhea, and syphilis more than 30 days after study enrollment were defined as incident STI cases. An incident case of chlamydia or gonorrhea was defined as a positive nucleic acid amplification test (NAAT) or culture on urogenital or extra-genital specimens; a subsequent new case was accepted as such if there was a positive test ≥30 days after the previous positive test. An incident case of syphilis was defined as having (i) a positive nontreponemal (NTr) titer of ≥1:8 with a previous nonreactive NTr; (ii) a four-fold increase in the NTr titer from the previous test; or (iii) positive treponemal test (Tr) if an NTr titer was ≥1:8 and the previous Tr test was negative. An STI episode included any combination of chlamydia, gonorrhea, and syphilis diagnosed on the same date.
We also evaluated frequency of STI testing. The total number of STI tests performed in the District of Columbia by year could not be determined because the linkage data from the DOH only included positive tests. We therefore used data on tests performed from the DC Cohort site data pre-linkage. For chlamydia, gonorrhea and syphilis, we calculated the number (percentage) of participants with one or more tests by calendar year, and the rate of testing per 100 P-Y defined as the total number of tests in the calendar year per 100 person years. Inclusion in the viral load analysis required at least one viral load by calendar year. Control of HIV among DC Cohort participants was examined using two methodologies: percentage durably suppressed (percentage with all viral loads less than the limit of detection (%<LLOD) for all measurements during the calendar year) and In-Care Viral Load (ICVL), the geometric mean of the means of viral loads measured during the calendar year among those in care with incident STIs.8 The LLOD can vary by site and year, or even within a year by a participant. For this analysis, we treated any LLOD value equally.
Statistical Analysis:
The incidence rate overall and stratified by socio-demographic variables for gonorrhea, chlamydia, syphilis, and any STI was calculated by dividing the number of STI episodes by total person time contributed in the analysis. Incidence rates were calculated per 100 person-years (P-Y) of observation, including all follow-up time contributed within each calendar year, using Rothman/Greenland estimation for 95% confidence intervals (CIs) and linear test of trend to examine changes over time.
The distribution of in-care viral load (ICVL) was non-normal. Therefore, the log10 of the mean in-care VL (ICVL) was calculated for each study participant by calendar year, and reverse transformed to original scale and presented as means (95% CI). The proportion of participants with VLs lower than the limit of detection was calculated for each calendar year, with a participant classified as lower than the LLOD, if all available viral loads were undetectable for each year. All statistical analyses were conducted using SAS Version 9.4 (Cary, NC). P-values <0.05 were considered statistically significant.
RESULTS:
During 2012–2016, 7,810 individuals were assessed for incident STI by calendar year. Among the 7,810 participants included in this analysis, 51.9% were included in four or more years of analysis, 36.1% in two to three years and 12.0% were included in one year of the analysis. The population included 71% cisgender male, 27% cisgender female, 2% transgender female; 1% aged 13–17, 20% aged 18–34, 54% aged 35–54, 25% 55 and older; 79% Non-Hispanic Black, 13% Non-Hispanic White, 6% Hispanic; 71% had permanent housing, 8% temporary/homeless; 64% had public insurance, 26% had private insurance; 49% MSM, 30% high risk heterosexuals (HRH), and 14% IDU.
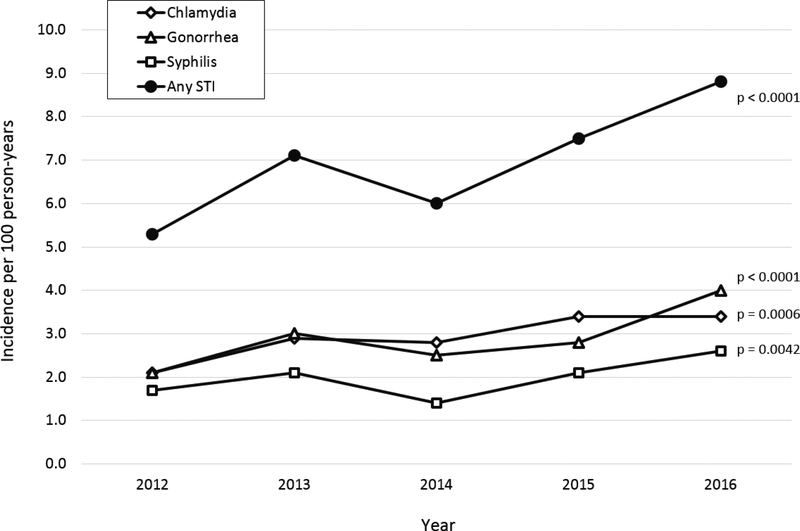
The incidence rates of all STIs all increased from 2012 to 2016 (Figure 1 with detail in Supplementary Table 1), including an increase in chlamydia from 2.1 to 3.4 cases per 100 person-years (p = 0.0006), gonorrhea from 2.1 to 4.0 cases per 100 person-years (p < 0.0001), syphilis from 1.7 to 2.6 cases per 100 person-years (p = 0.0042), and any incident STI episode from 5.3 to 8.8 cases per 100 person-years (p < 0.0001). The greatest relative increase was in gonorrhea, which in 2016 surpassed chlamydia as the most common incident STI in our study population. The proportion of those with incident STIs who had two or more STIs in the calendar year ranged from 14.0% to 21.6% for any STI episode, from 2.9% to 12.8% for chlamydia and from 11.7% to 14.3% for gonorrhea (Supplementary Table 2).
Figure 1: Incidence rates of sexually transmitted infections by year among DC Cohort participants, 2012–2016.
Incidence rates are presented per 100 person-years of follow-up.
P-values are based on a linear test of trend across the five years.
An STI episode may include any combination of chlamydia, gonorrhea, and syphilis diagnosed at the same date.
To address whether the increase in incidence could be explained by greater frequency of testing, we present data on the number of STI tests performed at the DC Cohort care sites (Supplementary Tables 3a to 3e). The number of chlamydia and gonorrhea tests and rate of testing per 100 P-Y rose sharply from 2012 to 2013, though continued to rise more gradually through 2016, for men, women and all race/ethnicities.
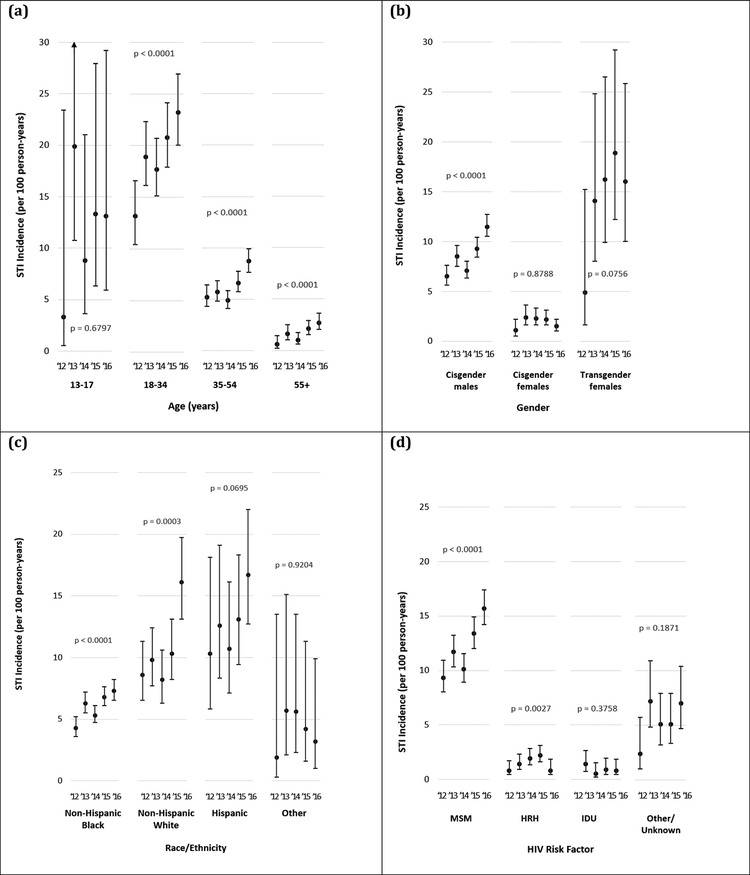
Trends in STI incidence among DC Cohort participants in 2012–2016 differed by baseline demographic characteristics (Figure 2 and Supplementary Table 3). An increase in STI incidence rates was observed across all adult age groups, with notable increases among participants aged 18–34 (from 13.2 to 23.2 cases/100 P-Y, p < 0.0001) and those aged 55 or older (from 0.6 to 2.7 cases/100 P-Y, p < 0.0001). Among participants aged 13–17, an increase from 3.3 to 13.1 cases/100 P-Y was observed (p = 0.6797; n = 109). STI incidence rates almost doubled among cisgender men (from 6.5 to 11.5 cases/100 P-Y, p < 0.0001), and increased though short of statistical significance among transgender women (from 4.9 to 16.0 cases/100 P-Y, p = 0.0756; n=138).
Figure 2: Incidence rates of sexually transmitted infections among DC Cohort participants in 2012–2016, stratified by baseline demographic characteristics: (a) age group, (b) gender, (c) race/ethnicity, and (d) HIV risk factor.
All demographics based on reporting at the time of DC Cohort enrollment.
STI incidence increased among all race ethnicities from 2012–2016, 8.6 to 16.1 cases/100 P-Y, p = 0.0003 for non-Hispanic Whites; 4.3 to 7.3 cases/100 P-Y, p < 0.0001 for non-Hispanic Blacks; 10.3 to 16.7 cases/100 P-Y, p = 0.0695 for Hispanics. STI incidence rates nearly doubled among participants reporting MSM-related acquisition of HIV (from 9.3 to 15.7 cases/100 P-Y, p < 0.0001) and tripled among those reporting HRH acquisition (0.8 to 2.4 cases/100 P-Y, p = 0.0027).
During 2012–2016, the ICVL among DC Cohort participants who had incident STIs decreased significantly overall from 108 copies/mL to 19 copies/mL (p <0.0001) and in specific subgroups (Table 1). ICVL also decreased significantly in all adult age groups, cisgender males and transgender females; all gender groups; non-Hispanic Blacks, and MSM, and those with 1 or 2 or more STIs.
Table 1:
In-Care Viral Load (ICVL) among DC Cohort participants with an incident STI (one or more) in 2012–2016, stratified by baseline demographic characteristics. ICVL shown in [COPIES/ML].
| In-Care Viral Load (SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012(n=123) | 2013(n=236) | 2014(n=247) | 2015(n=299) | 2016(n=296) | P | ||||||
| Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | N | ||
| Overall: | 108 (1,54173) | 123 | 54 (1,61638) | 236 | 32 (1,55823) | 247 | 24(1,33480) | 299 | 19 (1,60743) | 296 | |
| Age at enrollment: | |||||||||||
| 13–18 | 3064 (3064,3064) | 1 | 686 (1,111848) | 9 | 663 (10.5,16731) | 4 | 3018 (5.75,26259) | 5 | 56 (1,11645) | 4 | 0.3579 |
| 18–35 | 211 (1,103972) | 51 | 101 (1,78134) | 111 | 43 (1,63896) | 121 | 51 (1,44111) | 128 | 35 (1,81491) | 116 | <0.0001 |
| 35–54 | 64 (1,30347) | 68 | 30 (1,25938) | 99 | 27 (1,22670) | 108 | 12 (1,12759) | 135 | 15 (1,40866) | 145 | <0.0001 |
| 55+ | 59 (1,684.2) | 3 | 9 (1,71814) | 17 | 3 (1,51513) | 14 | 8 (1,5167.5) | 31 | 6 (1,12615) | 31 | 0.0005 |
| Gender: | |||||||||||
| Cisgender M | 103 (1,80948) | 114 | 50 (1,61638) | 202 | 24 (1,55823) | 204 | 16 (1,14740) | 258 | 15 (1,41419) | 264 | 0.0003 |
| Cisgender F | 155 (1,4357.7) | 7 | 77 (1,46745) | 22 | 89 (1,57650) | 28 | 583 (1,73542) | 25 | 370 (1,292357) | 19 | 0.0656 |
| Transgender F | 524 (94.2,2910) | 2 | 113 (1,23447) | 12 | 176 (1,20664) | 15 | 94 (1,40470) | 16 | 53 (1,257574) | 13 | 0.0036 |
| Transgender M | NA (NA) | 0 | NA(NA) | 0 | NA(NA) | 0 | NA(NA) | 0 | NA(NA) | 0 | NA |
| Race/Ethnicity: | |||||||||||
| Non-Hisp. Black | 226 (1,80948) | 81 | 77 (1,61638) | 166 | 53 (1,63716) | 177 | 39 (1,40105) | 218 | 29 (1,60743) | 204 | <0.0001 |
| Non-Hisp. White | 41 (1,19524) | 33 | 29 (1,71814) | 51 | 9 (1,12469) | 45 | 4 (1,6560) | 52 | 6 (1,105820) | 55 | <0.0001 |
| Hispanic | 6 (1,88) | 8 | 11 (1,15187) | 16 | 7 (1,1372.9) | 20 | 12 (1,29269) | 27 | 13 (1,32325) | 36 | 0.0325 |
| Other | 1 (1,1)) | 1 | 67 (20,591.33) | 3 | 21 (1,2029.5) | 5 | 417 (168.67,1030) | 2 | 1 (1,1) | 1 | 0.2960 |
| Unknown | NA (NA) | 0 | NA(NA) | 0 | NA(NA) | 0 | NA(NA) | 0 | NA (NA) | 0 | NA |
| Insurance: | |||||||||||
| Private | 60 (1,103972) | 36 | 19 (1,32534) | 64 | 12 (1,55867) | 73 | 7 (1,16540) | 92 | 9 (1,79240) | 77 | <0.0001 |
| Public | 119 (1,54173) | 72 | 71 (1,46745) | 124 | 57 (1,57650) | 145 | 46 (1,39045) | 176 | 21 (1,40866) | 193 | <0.0001 |
| Other/Unknown | 282 (1,1040000) | 15 | 113 (1,129220) | 48 | 18 (1,16175) | 29 | 20 (1,6484.2) | 31 | 88 (1,124930) | 26 | 0.0021 |
| Housing: | |||||||||||
| Permanent | 114 (1,82160) | 85 | 44 (1,50410) | 164 | 27 (1,45630) | 181 | 20 (1,26259) | 219 | 18 (1,60743) | 224 | <0.0001 |
| Temp./Homeless | 157 (1,17577) | 17 | 75 (1,29633) | 24 | 52 (1,100939) | 31 | 40 (1,44111) | 42 | 9 (1,56060) | 35 | <0.0001 |
| Other/Unknown | 65 (1,30584) | 21 | 95 (1,67677) | 48 | 47 (1,57650) | 35 | 36 (1,40105) | 38 | 58 (1,257574) | 37 | 0.1822 |
| Risk for HIV: | |||||||||||
| MSM | 114 (12.8,3064) | 112 | 52 (1,61638) | 194 | 28 (1,55823) | 201 | 17 (1,14740) | 248 | 16 (1,60743) | 247 | <0.0001 |
| HRH | 32 (1,4357.7) | 6 | 10 (1,3477.5) | 16 | 63 (1,54167) | 25 | 60 (1,88145) | 27 | 77 (1,275735) | 28 | 0.0004 |
| IDU | NA (NA) | 0 | 66 (1,2878.7) | 8 | 71 (1,2200.6) | 4 | 200 (1,9946.3) | 6 | 5 (1,24610) | 6 | 0.9376 |
| Other/Unknown | 115 (1,80948) | 5 | 333 (1,111848) | 18 | 41 (1,65939) | 17 | 263 (1,53436) | 18 | 48 (1,40866) | 15 | 0.6190 |
| STI Diagnosis: | |||||||||||
| 1 | 102 (1,80948) | 107 | 54 (1,61638) | 199 | 25 (1,41810) | 210 | 23 (1,40105) | 235 | 23 (1,60743) | 231 | <0.0001 |
| 2+ | 163 (1,19524) | 16 | 60 (1,71814) | 37 | 129 (1,80603) | 37 | 27 (1,25496) | 64 | 11(1,41419) | 65 | 0.0163 |
CI, confidence interval; NA, not applicable (i.e., zero cases; insufficient data for calculation); MSM, men who have sex with men; HRH, high risk heterosexual; IDU, injection drug use. P-values are based on a linear test of trend across the five years.
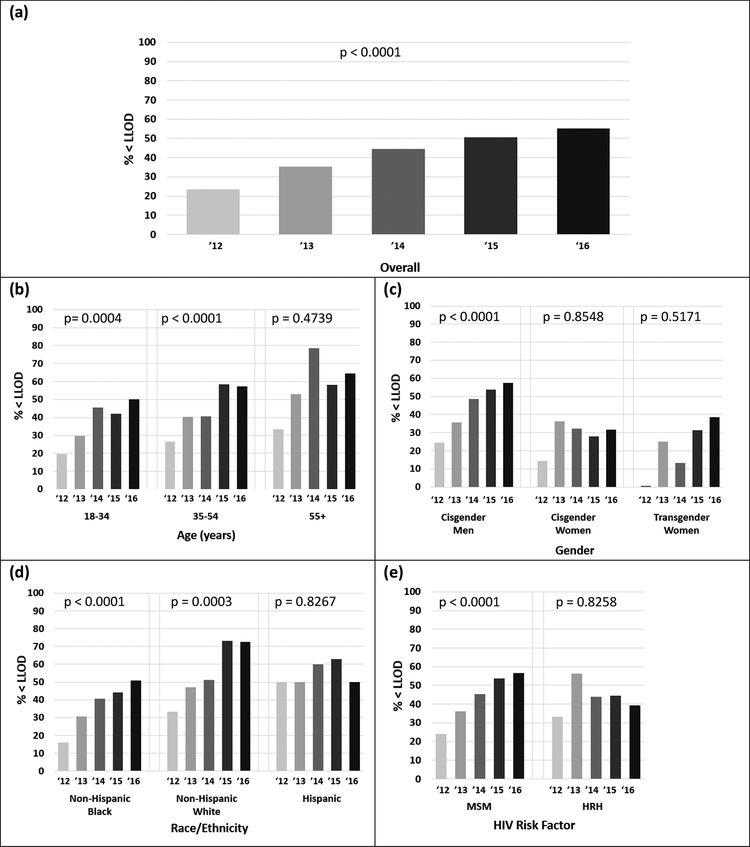
From 2012 to 2016 the percentage of individuals with incident STIs with all viral loads in the calendar year less than the limit of detection (LLOD) also improved substantially; from 23.6% in 2012 (n = 123) to 55.1% in 2016 (n = 296) (Figure 3). By 2016, notable differences remained in both ICVL and % < LLOD among 13–18 year olds (ICVL: 56; % < LLOD: 50%) and 18–35 year olds (ICVL: 35; % < LLOD: 50%) compared to older age groups 55+ (ICVL: 6; % < LLOD: 64.5%); among cisgender females (ICVL: 370; % < LLOD: 31.6%) and transgender females (ICVL: 53; % < LLOD: 38.5%) compared to cisgender males (ICVL: 15; % < LLOD: 57.56) and among non-Hispanic Blacks (ICVL: 29; % < LLOD: 51.0.%) and Hispanics (ICVL: 13; % < LLOD: 50%) compared to non-Hispanic Whites (ICVL: 6; % < LLOD: 72.7%).
Figure 3: Percentage with all viral loads below the limit of detection (%<LLOD) 2012–2016 among those with at least one STI in the calendar year, stratified by category as follows: (a) overall, (b) age, (c) gender, (d) race/ethnicity, and (e) HIV risk factor.
Participants whose race/ethnicity and HIV risk factor were unknown are excluded, as are those ages 13–17 and those who reported IDU as their risk factors are excluded due to low sample size.
DISCUSSION:
Our results demonstrate a notable rise in incident STIs among PLWH enrolled in the DC Cohort from 2012 to 2016, with frequent occurrence of 2 or more STIs in a calendar year. These trends mirror those reported among DC residents overall, and among the general US population in the same time period. On the other hand, two measures of HIV viral load over each calendar year improved consistently across all groups, likely associated with reduced HIV transmission risk for many unprotected exposures marked by an incident STI. Nevertheless, differences across demographic groups remain, demonstrating the need to focus services on PLWH who may be particularly vulnerable to worse outcomes.
Though, we saw an increase in STI testing frequency for chlamydia and gonorrhea between 2012 and 2016, we do not believe this fully explains the increase in STI incidence. Increased testing likely reflected increase in both screening in asymptomatic patients, and an increased number of patients presenting with symptoms or with known STI exposures.
Reduction of incident STIs has proven difficult. Condom use as the sole primary prevention method has historically failed to achieve high efficacy rates.9 Yet, in the era of U=U (Undetectable = Untransmittable), improved messaging around high STI rates and HIV acquisition risk remain of importance toward reducing high risk behaviors. Additional secondary prevention strategies include post-exposure prophylaxis (PEP) for STIs, self- and point-of-care testing, home-based sample collection, and technology-based interventions such as mobile applications promoting sexual health and internet-based partner notification systems.2,10 Furthermore the importance of promoting evidence based guidance to clinicians to improve screening for STIs cannot be overstated.11
Although all adult (age 18+) age groups saw an increase in STI incidence rates during the study period, there are important implications of the differences in STI incidence by demographic subgroups within the city. Eighteen to thirty-four year olds had an approximately ten-fold risk of incident STIs compared to those aged 55 or older. While among cisgender women STI incidence rates remained low and nearly constant, among cisgender men and transgender women they approximately doubled. Participants who reported HIV acquisition through sexual contact (both MSM and HRH) had statistically significant increases in STI incidence during the study period, though the change was more pronounced among MSM. Additionally, the absolute incidence among MSM was 5–6 fold that of HRH. Such findings have previously been linked to an increase in high-risk sexual activities, such as sexual connection with multiple partners within a short period of time via mobile applications, forgoing condoms, and engaging in “chemsex” or the consumption of drugs to facilitate sexual activity.12,13
Although, non-Hispanic Blacks made up a majority of the DC Cohort population, they had a lower incidence of STIs through all calendar years, compared to Hispanics (who had the highest incidence) and non-Hispanic Whites. These findings differ somewhat from national figures, in which Blacks made up a majority of individuals accounting for chlamydia, gonorrhea, and syphilis.11 Typically, STI incidence has been shown to be higher in ethnic and minority groups due to higher poverty rates, unemployment, education, and lack of access to healthcare in addition to stigma, depression, substance abuse and decreased self-efficacy.2,11 However, a lower rate of STIs among Non-Hispanic Blacks has been consistent across this and a prior DC Cohort analysis.5 The reason for this unexpected finding in Washington DC is uncertain, but important and an area for future research.
Despite the noted rise in STI incidence among all groups, there was a decrease over time in two measures of viral control, in care viral load (ICVL) and percentage less than the lower limit of detection (%<LLOD) across all populations. When considering the populations with both the highest number of STIs and the highest incident rates of STIs, these notable improvements between 2012 and 2016 took place among 18–54 year-olds, MSM, non-Hispanic Blacks, cisgender males, and even among those with 2 or more STIs in the calendar year. Although these findings are quite encouraging, the data highlights the challenges of achieving viral suppression and eliminating HIV transmission risk; durable viral suppression during 2016 was achieved only by 55% of those with STIs overall. Inequities were apparent with the lowest percentage suppressed in 2016 among 18–35 year-olds (50%) compared to 55+ (65%), cisgender females (31.6%) and transgender females (38.5%) compared to cisgender men (57.6%) and non-Hispanic Blacks (51.0%) compared to non-Hispanic Whites (72.7%).
Ongoing efforts in the District of Columbia to engage patients in care and achieve viral suppression at sites of care and via the DC Department of Health have proven to be meaningful. In an effort to reduce the incidence of STIs and HIV, in 2017 the DC Department of Health supported 95,334 HIV tests, distributed more than 5.2 million male and female condoms, supported more than 1,700 persons to obtain PrEP, removed 592,853 needles from the street, provided free STI testing for more than 5,000 young people, and provided HIV medical care and support services to 8,000 persons through the Ryan White CARE Program.4 The nationwide endorsement of U=U (Undetectable = Untransmittable)14, along with findings from the PARTNER-1, PARTNER-2 and Opposites Attract studies (an HIV transmission rate of zero between serodiscordant couples who engage in condomless sex when HIV viral load is suppressed), have helped to alleviate the stigma attached to HIV.15,16 Yet, our analysis highlights the complexity of the path toward reduced STIs, achievement of durable HIV suppression, a reduction in new HIV infections and ultimately ending the HIV epidemic.
This analysis combines citywide data on STI incidence and longitudinal HIV viral suppression, capturing sexual transmission risk over time, but has several important limitations. We were not able to determine if STIs were found at the time of routine screening or in the setting of clinical symptoms. Furthermore, STI screening practices are not standardized across the cohort and it is possible that this variability in testing had an impact on our findings. Additionally, a new chlamydia or gonorrhea diagnosis was defined as a positive result at least 30 days after a prior positive; treatment of the prior episode was assumed but not confirmed. On the other hand, our stringent laboratory definition of syphilis may have excluded some cases of latent and tertiary syphilis, likely resulting in under-estimation for this disease.
In conclusion, our findings suggest that though a rise in incident STIs, district-wide initiatives on ART initiation and adherence support are making an impact for HIV control and transmission potential among people with STIs. Further investigation is needed to determine if this improvement in viral suppression offsets the increased risk of HIV sexual transmission in the setting of rising STI rates in the same population. In order to meet the goals of the U.S. Department of Health and Human Services’ Ending the HIV Epidemic initiative, further efforts are needed to bring the number of new HIV transmission events in DC down to zero. Innovative strategies will be needed to achieve HIV suppression among all those living with HIV infection and to enhance timely HIV and STI prevention, focusing on the groups that bear the greatest risks.
Supplementary Material
KEY MESSAGES:
The rise in STIs among PLWH in Washington DC has mirrored the overall rise in STIs in the U.S. and many urban centers around the world.
At the same time, our estimates of HIV transmission potential have improved considerably by year, though inequities are evident by population.
These findings highlight the work to be done in achieving durable viral suppression toward improved HIV outcomes and ending the HIV epidemic; AND the importance of PrEP.
ACKNOWLEDGMENTS:
We would like to thank the site Principal Investigators, Research Assistants, the Community Advisory Board, the patients themselves, the DC Department of Health, and the National Institutes of Health (NIH) for their contributions to the DC Cohort. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Data in this manuscript was collected by the DC Cohort Executive Committee with investigators and research staff located at: Cerner Corporation (Jeffery Binkley, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, Qingjiang Hou, Jeff Naughton, David Parfitt); Children’s National Medical Center Adolescent (Lawrence D’Angelo) and Pediatric (Natella Rahkmanina) clinics; The Senior Deputy Director of the DC Department of Health HIV/AIDS, Hepatitis, STD and TB Administration (Michael Kharfen); Family and Medical Counseling Service (Michael Serlin); Georgetown University (Princy Kumar); The George Washington University Medical Faculty Associates (David Parenti); The George Washington University Department of Epidemiology and Biostatistics (Alan Greenberg, Maria Jaurretche, Brittany Wilbourn, James Peterson, Morgan Byrne, Yan Ma); Howard University Adult Infectious Disease Clinic (Ronald Wilcox), and Pediatric Clinic (Sohail Rana); La Clinica Del Pueblo, (Ricardo Fernandez); MetroHealth (Annick Hebou); National Institutes of Health (Carl Dieffenbach, Henry Masur); Providence Hospital (Jose Bordon); Unity Health Care (Gebeyehu Teferi); Washington Hospital Center (Maria Elena Ruiz); and Whitman-Walker Health (Deborah Goldstein). FUNDING: The DC Cohort is funded by the National Institute of Allergy and Infectious Diseases, UO1 AI69503-03S2. This research was supported by the District of Columbia Center for AIDS Research, an NIH funded program (AI117970), which is supported by the following NIH CoFunding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH,NIA, FIC, NIGMS, NIDDK, and OAR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. IRB APPROVAL: #071029 George Washington University IRB
Footnotes
Potential conflicts of interest. All authors report no potential conflicts.
Prior presentation. Portions of this analysis were presented during ID Week’s Poster Abstract Session on October 5, 2018, under the title “Sexually Transmitted Infections among Persons Living with HIV Infection and Receiving Care in the District of Columbia: Time with Viral Load above 1500 as Proxy for Risk of Transmission” (Poster No. 1498) and at CROI in March 2019, under the title “Incident STIs among PLWH in Washington, DC: Measuring HIV transmission risk” (Poster No. 849).
REFERENCES:
- 1.Centers for Diseases Control and Prevention. STD diagnosis among key US populations: 5-year trends. CDC; 2018. [Google Scholar]
- 2.Mayer KH and de Vries H. HIV and sexually transmitted infections: responding to the newest normal. Journal of the International AIDS Society 2018, 21:e25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually Transmitted Diseases. 1992;19(2). [PubMed] [Google Scholar]
- 4.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, and TB Administration. Annual Epidemiology & Surveillance Report – Data Through December 2017.
- 5.Lucar J, Hart R, Rayeed N, et al. Sexually Transmitted Infections Among HIV-Infected Individuals in the District of Columbia and Estimated HIV Transmission Risk: Data From the DC Cohort. Open Forum Infect Dis. 2018; 5: ofy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohort DC Longitudinal HIV Study. http://go.gwu.edu/dccohort. Accessed February 19th, 2019.
- 7.Greenberg AE, Hays H, Castel AD, et al. Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: Technical challenges and innovative solutions. J Am Med Inform Assoc. 2016;23(3):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon SS, Mehta SH, McFall AM, et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: A cross-sectional, comparative study. The Lancet HIV. 2016;3(4):e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low N, Broutet NJ (2017) Sexually transmitted infections—Research priorities for new challenges. PLoS Med 14(12): e1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina JM, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis 2018; 18:308–17. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Diseases Control and Prevention. Sexually Transmitted Diseases Surveillance 2016.
- 12.Brooks JT, Buchacz K, Gebo KA, et al. HIV infection and older Americans. The public health perspective. Am J Public Health. 2012;102: 1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pufall EL, Kall M, Shahmanesh M, et al. Sexualized drug use (‘chemsex’) and high-risk sexual behaviours in HIV-positive men who have sex with men. HIV Med. 2018;19(4):261–270. doi: 10.1111/hiv.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. https://www.preventionaccess.org/
- 15.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bavinton BR, et al. Viral Suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018. August;5(8):e438–e447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.