Figure 9. Model of MRP126 Ca(II)-dependent Zn(II) binding.
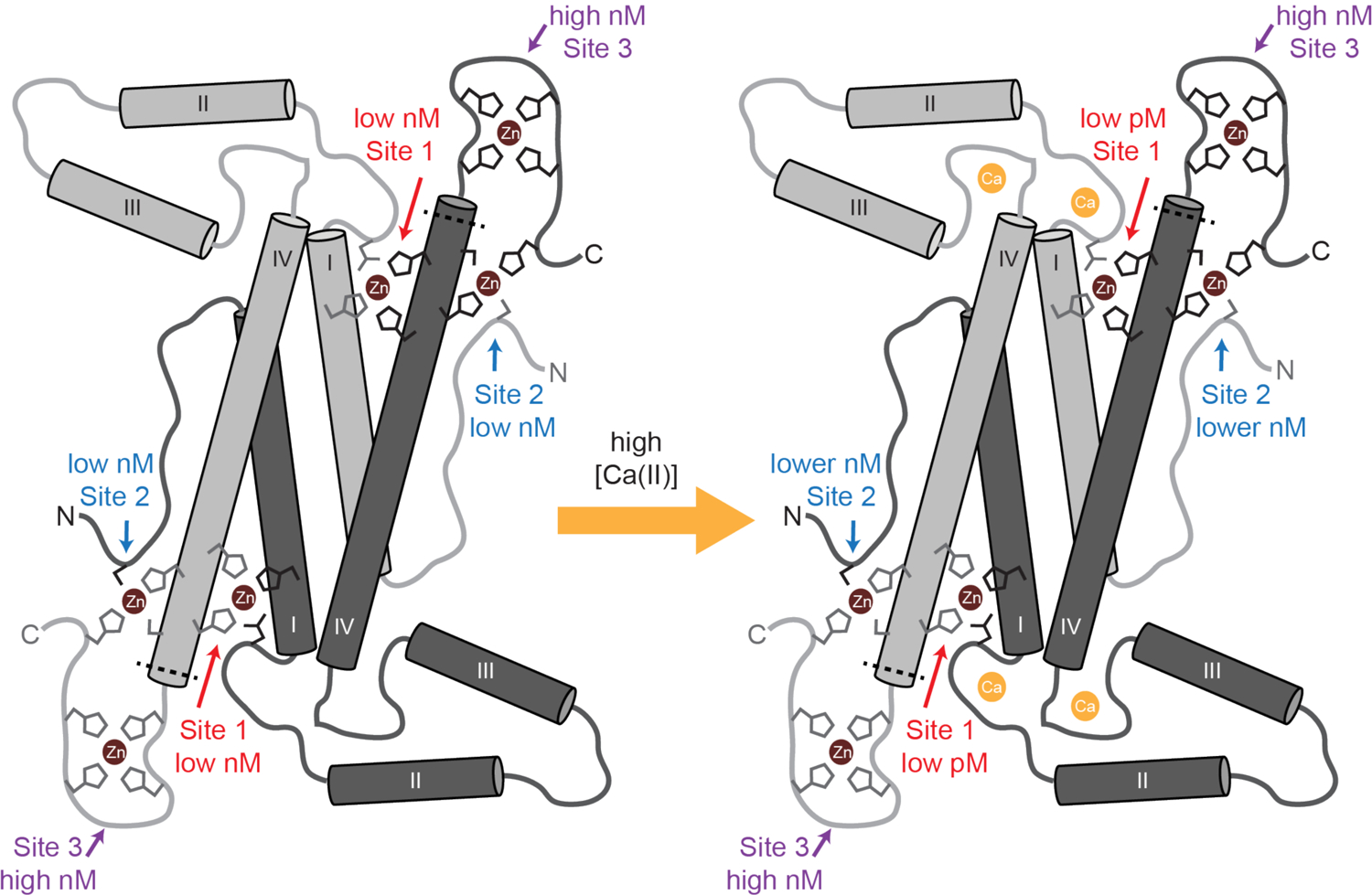
Each MRP126 homodimer contains three distinct pairs of Zn(II)-binding sites. Site 1 is a canonical calgranulin His3Asp site comprised of His26 and Asp36 from one monomer and His96 and His100 from the other monomer. Site 2 is a dicysteine site that likely consists of Cys5 from one monomer and Cys102, His98, and a C-terminal tail histidine from the other monomer, which we denote Cys2His2. Site 3 likely consists of multiple histidines from the C-terminal tail of one monomer, which we call Hisx. In the absence of Ca(II), Zn(II) binds to MRP126 with similar low-nanomolar affinity at either Site 1 (His3Asp) or Site 2 (Cys2His2), then with high-nM affinity at Site 3 (Hisx). Ca(II) dramatically increases the affinity of Site 1 and increases the affinity of Site 2 to a lesser degree. Thus in the presence of Ca(II), Zn(II) preferentially binds with low-pM affinity to Site 1 (His3Asp), then with lower-nanomolar affinity to site Site 2 (Cys2His2), and finally with high-nanomolar at Site 3 (Hisx). The primary coordination sphere for the Hisx site is unknown and it is drawn as a His4 site. The dashed line indicates the approximate location of the point of truncation for the ΔTail variant.
