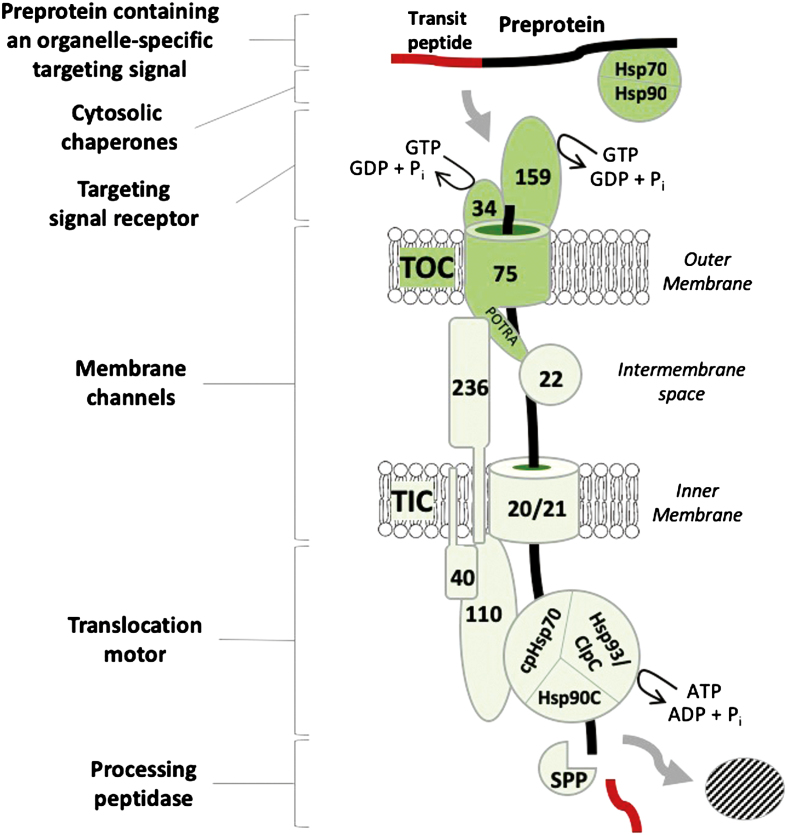
Fig. 1.
The core components of the TOC–TIC general import machinery of chloroplasts. The core components of the general import machinery are conserved across the green lineage. The newly synthesized preprotein is targeted to the TOC complex at the outer membrane by binding of its intrinsic transit peptide to the Toc34 (34) and Toc159 (159) receptors. Targeting is aided by cytosolic chaperone complexes of the Hsp70 and Hsp90 families. The GTPase activities of the receptors function as a checkpoint for the commitment of the preprotein to transport through the TOC and TIC membrane channels formed by Toc75 (75) and Tic20/21 (20/21), respectively. TOC–TIC supercomplexes formed by the binding of Tic236 (236) to Toc75 and Tic110 (110) at the TIC complex facilitates direct transport of the preprotein from the cytoplasm to the stroma. Mistargeting to the intermembrane space is avoided by the combined chaperone activities of the Toc75 POTRA domains and Tic22 (22). Tic20/21 form the major components of the TIC import channel, and they associate with Tic110 and Tic40 (40), which form a scaffold for the assembly of the ATP-dependent import motor in the stroma. The import motor drives unidirectional translocation of the preprotein via the combined activities of cpHsp70, Hsp93/ClpC, and Hsp90C chaperones. Upon import, the transit peptide is cleaved by the stromal processing peptidase (SPP). The reader is referred to other recent reviews for more comprehensive descriptions of accessory components associated with the general import machinery.