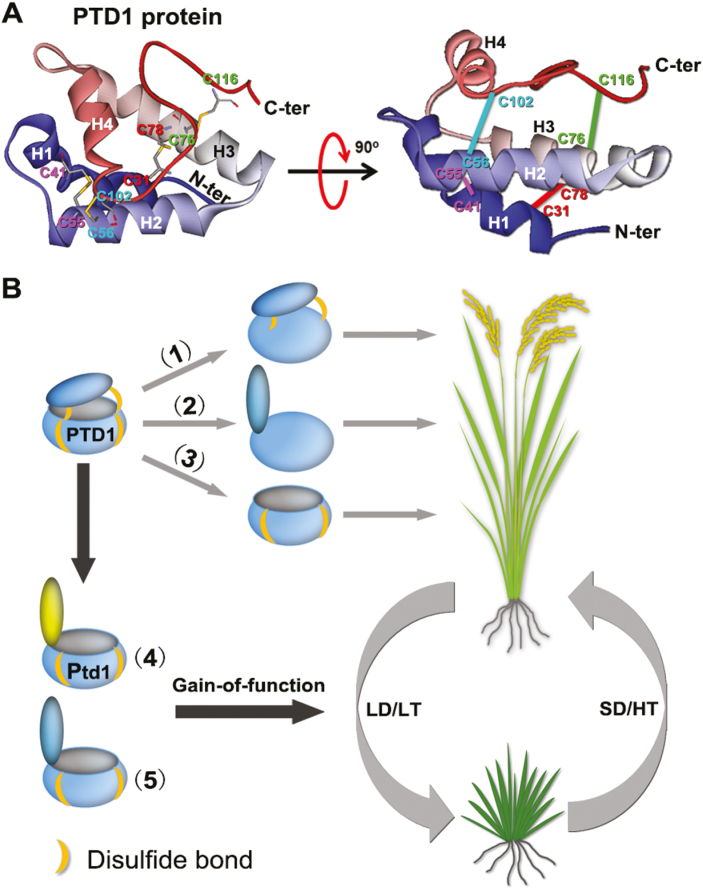
Fig. 7.
Gain-of-function of rice Ptd1 may be due to the specific conformation of the protein. (A) Predicted 3D structure of PTD1 (excluding the 27-aa signal peptide in the N terminus). Left, top view. The positions of the conserved Cys residues are indicated by ‘C’ followed by a number. The pairs C31–C78, C41–C55, C56–C102, and C76–C116 each form a specific disulfide linkage (shown by the connecting bars). Right, side view. The straight lines correspond to the disulfide linkages shown in the top-view. H1–H4, indicate the four alpha helixes. C-ter and N-ter are C and N termini, respectively. (B) Gain-of-function of Ptd1 may be due to the specific protein conformation. The PTD1 protein forms a hydrophobic pocket structure with a C-terminal lid, consolidated by four disulfide bonds. Loss of the lower two (1) or all four (2) disulfide bonds, or loss of the C terminus (3) has no biological effect on plant growth (i.e. no inhibition). In contrast, the mutated protein Ptd1 (4), which is expressed under low-temperature (LT) and/or long-day (LD) conditions, loses the upper two disulfide bonds that screw the lid and the pocket, but retains the lower two disulfide bonds for pocket-fixing. This protein conformation may form a molecular ‘catcher’ or ‘trap’ and thus the protein gains a biological function for growth inhibition. The conformation in (5) represents the artificially mutated PTD1 forms in the transgenic plants (PTD1C102G/C116G and PTD1C56G/C76G; Fig. 6B, C). Under high-temperature (HT) and short-day (SD) conditions, the expression of Ptd1 is at relatively low levels (Fig. 3E, F) and hence plant growth is recovered. (This figure is available in color at JXB online.)