Abstract
Due to the longer survival of critically ill children, venous thromboembolism is a problem which is becoming increasingly recognized in pediatric practice. In the last decades, several international studies have been published, shedding a light upon the epidemiology of this disease during childhood. These data show peculiarities in the clinical presentation and the significant morbidity and mortality. The new “epidemic of thrombosis” in pediatric hospitals points toward the urgent need for specific treatment and prevention protocols targeting this population. In Brazil, knowledge regarding this disease remains scarce. The lack of epidemiological data impacts both the clinical care and the design of specific public policies in the field. Thus, a national registry of pediatric venous thromboembolism is relevant to the proposal of an appropriate plan of action to create a qualified net of assistance. The improvement in educational initiatives related to the field of Pediatric Hemostasis is also very important. In this review, we have updated the epidemiological, clinical and therapeutic aspects of the disease, as well as the prevention strategies.
Keywords: Venous thrombosis, Children, Epidemiology, Risk factors
Introduction
Until recently, venous thromboembolism (VTE) during childhood was considered a rare event. This concept has currently changed due to existing data, which shows a significant increase in reported cases, as well as the high associated morbidity and mortality rates. In 1994, a study group from Canada reported for the first time a prospective study of VTE in children.1 This was the starting point for several countries to publish their data from studies, fundamental for the consolidation of the portrayal of this disorder in childhood. However, the majority of these VTE studies in childhood were limited to predominantly Caucasian populations in the United States and European countries.
In 2006, pediatric hospital data banks in the United States reported over 4500 cases of VTE in Children.2 This data is almost ten times higher than the amount reported by Andrew et al. in the first registry of the disease in the 1990s.1
Differences in the genetic profile and demographic aspects are known to influence the clinical presentation of VTE. Data from adults from distinct ethnic groups, including those in Brazil, point toward peculiarities in the epidemiology of the thrombotic event, when compared to caucasoides.3, 4
Twenty years have elapsed since these pioneer studies and Brazil remains without a registry of pediatric VTE cases and consequently, with no knowledge regarding the epidemiological situation in the country, nosology and local variations. This lack of data has led us to include the subject in a Thematic Project on VTE funded by Fundação de Amparo à Pesquisa de São Paulo – FAPESP, the Sao Paulo Research Foundation, which we believe will enable us to outline the epidemiologic profile in our country.
The aim of this paper is to update the epidemiological, clinical, diagnostic and therapeutic aspects of childhood VTE, as well as prevention strategies.
Epidemiology
From the 1990s forward, the first epidemiological studies on VTE during childhood were carried out.1, 5, 6, 7, 8 The first report on VTE revealed an incidence of 0.07/10,000 children in Canada.1 Later Holland and United Kingdom published data on the yearly incidence of 0.14 and 0.07 out of 10,000 children, respectively.5, 6 Among neonates, the incidence registered was 24 cases for every 10,000 admissions to the neonatal intensive care unit in Canada7 and of 0.5 out of 10,000 born in Germany.8 Data from North American hospitals in 2000 demonstrated approximately 20 cases per 10,000 admissions.2, 9
Another study restricted to children hospitals pointed toward a dramatic increase in pediatric VTE incidence over time. The annual rate increased 70%, from 34 to 58 cases per 10,000 hospitalizations from 2001 to 2007.10 Apparently, this increase was confined to the tertiary units, as the populational studies maintained the stable historical series of pediatric VTE throughout the country.11 This situation is believed to be the result of the improved diagnosis and surveillance, technological advancement in the treatment of severely ill children, growing use of vascular devices and higher survival of these children.12
It is important to consider the difference in the design of each study when interpreting the results. Prospective and multicentric studies were performed in the 1990s,2, 5, 6, 7, 8 contrary to those described in the following decades, which included the retrospective analysis of hospital databases, using International Disease Code to identify the cases.1, 9, 10
These studies help elucidate specific features of the disease in childhood (Table 1).
Table 1.
Epidemiological–clinical aspects of venous thromboembolism in children compared to adults.
| Childhood (0–18 years of age) | Adults | |
|---|---|---|
| Incidence | 0.07–0.5/10.0002, 5, 6, 11 PEa: 0.09/10.00011 |
5.6–16/10.00034 PEa: 3–7.8/10,00049 |
| Age | 2 peaks: first year of life and in adolescence11 | Exponential increase with age49 |
| Sex (M:F) | 1:12, 5, 15 | Female predominance in childbearing years and male predominance > 45 years old49 |
| Ethnicityb | Greater incidence in African descendents11 | Greater incidence in African descendents and lower in Asians3 |
| Idiopathic thrombosis | 2–8%2, 5, 6, 9, 13, 19 | 25–40%49 |
| Location | ||
| Upper limbs | 0–60%2, 5, 6, 13, 15, 19 | 10%50 |
| Lower limbs | 36–65%2, 5, 6, 13, 19 | 80%50 |
| Recurrence | 5.5–21%2, 5, 6, 9, 15, 19, 21 | 30%49 |
| Death | 9–20%2, 5, 6, 9, 15, 20 | 5%49 |
| Postthrombotic syndrome | 12–70%2, 15, 20 | 20–50%34 |
Pulmonary embolism.
Compared to Caucasians.
The neonatal period represents 50% of the total amount of VTE.8 Regarding gender, some studies have shown a male predominance among infants during the first year of age and a female predominance during adolescence, associated with hormonal factors, such as contraceptive pill intake and pregnancy.11, 13
Most studies on adults show that limb thrombosis is predominant in the lower limb venous system, compared to the upper limb venous system. Nevertheless, thrombosis of the upper extremities is more common in children than in adults (see Table 1). This fact has been associated with the use of a central venous catheter (CVC), which is usually placed in the upper limbs.
Etiopathogeny
As a consequence of developmental hemostasis, children are protected against thrombosis, and one of the main reasons is the greater integrity of the vascular endothelium, unaffected by vascular comorbidities. Specific features of the hemostatic system during the first months of life are considered protection factors: lower plasma concentration of coagulation factors and elevated levels of α2-macroglobulin with consequent thrombin inhibition.14
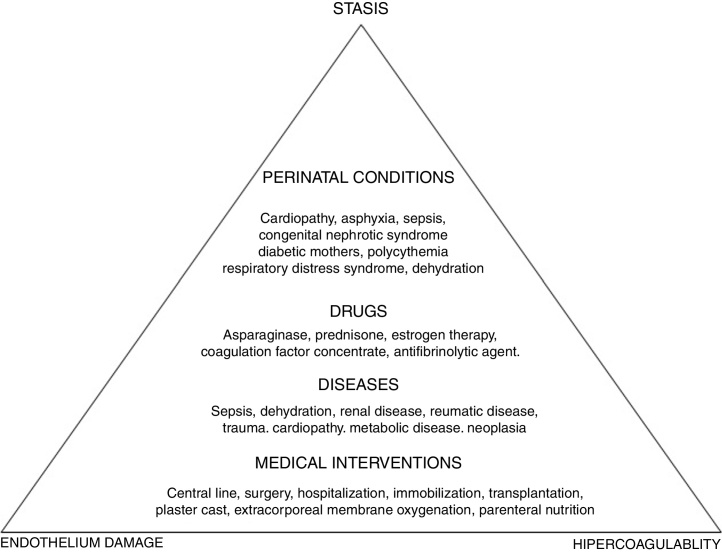
To better understand the pathogenesis of the disease, a look at the Virchow's triad is imperative, as this triad describes the three mechanisms involved in the disorder: vascular endothelium damage, blood stasis and hypercoagulability state. Ninety percent of the cases of childhood VTE are provoked, that is, are associated with triggering factors (Figure 1).
Figure 1.
Virchow's triad and risk factors for pediatric venous thromboembolism.
According to international pediatric registries, the risk factors for thromboembolic events in children are the following: CVC, trauma, cancer, cardiopathies, infections and nephropathies.1, 5, 6, 7, 8, 15 The CVC appears first on the list and is responsible for 33–60% of the thrombotic events.1, 5, 6, 7, 8, 15 In older children and adolescents, the risk factors are similar to the risk factors in adults and are related to trauma, contraceptive pill intake, surgery and obesity.16, 17
The incidence of VTE in oncology is 2–8%. The types of cancer at greater risk are acute lymphoid leukemia (ALL), lymphoma, and sarcomas. The main risk factors are the CVC, present in one third of these thromboses, and the use of certain chemotherapy agents, such as l-asparaginase, associated with high doses of corticosteroids, which reduce the levels of antithrombin and increase the levels of factor VIII, thus leading to an increase in the generation of thrombin. In addition, there are specific pathology factors; in sarcomas, the presence of metastatic disease and in lymphomas, a mediastinal mass.18
Thrombophilia is the hereditary or acquired tendency for thrombosis, and the prevalence of the former is 13–60%.19, 20, 21 The higher rates are related to a cohort of older children with spontaneous episodes19 who have a positive family history for the disease, which is considered a predicting factor for test positivity.20
A meta-analysis demonstrated expressive influence of hereditary thrombophilia as a risk for the first event, with a relative risk from 2.2 for the G202010A mutation in the prothrombin gene to 9.4 for antithrombin deficiency. Furthermore, except for Factor V Leiden, all other thrombophilia was associated with a significant risk of recurrence, though lower when compared to the first episode.22 Regarding VTE associated with the use of a catheter, no relevant role was demonstrated for hereditary thrombophilia.23
The antiphospholipid antibody syndrome (APS) is characterized by arterial or venous thrombosis in the presence of persistent antiphospholipid antibodies. The records of pediatric APS show that 50% of the patients have an associated autoimmune disorder, mainly lupus, and in 60% of the cases, the thrombus is located at the venous site. The risk of recurrence is 20%, a higher rate than in adults.24 A specific aspect in childhood is the high frequency of non-thrombotic manifestations, such as cytopenias, migraine, epilepsy and chorea, which are more prevalent in APS secondary to an underlying autoimmune disease. Whether these symptoms are related to APS or to the underlying autoimmune disorder remains unclear.24
As the majority of the cases of childhood VTE is provoked by multiple factors, it is plausible to infer in clinical practice that the role of hereditary thrombophilia is less relevant in the occurrence of the primary episode. Thus, the current tendency is to avoid indiscriminate screening,25, 26 except for spontaneous events or a family history consistent with VTE, in which case investigation should be considered.27
Clinical manifestations
The clinical expression depends on the site of the disorder; however there are some aspects which are characteristic during childhood. Purpura fulminans is a rare syndrome caused by protein C or protein S deficiency, characterized by progressive cutaneous hemorrhagic necrosis in neonates.27 Renal vein thrombi, more predominant among males, manifest themselves during the first days of life, along with hematuria, thrombocytopenia and a palpable abdominal mass.28
Thrombosis associated with the CVC, has a silent evolution. Data point to the fact that for each symptomatic case, eight cases are asymptomatic, and can only be identified by radiological screening before device removal.29 In spite of this, clinical signs can be used to identify CVC thrombosis. In neonates symptoms include loss of patency, persistent sepsis or thrombocytopenia. Swelling in the affected limb only occurs in 10–36% of the cases.30 These findings differ from those of older children, for whom limb edema is frequent and thrombocytopenia is a rare event.31
During the neonatal period, portal vein thrombosis, associated with umbilical catheterization, may lead to abdominal distension, thrombocytopenia and an increase in transaminases. In more than 50% of the cases, the resolution is spontaneous. An intracardiac thrombus when small is often asymptomatic, however embolization can lead to migration to the lungs causing pulmonary embolism (PE), or to systemic circulation with cerebral ischemia in the case of patients born with patent foramen ovale.30
Thrombosis of the limbs usually presents with pain, erythema, edema of the affected limb and, when affecting an upper extremity, may cause the superior vena cava syndrome (SVC).31 In PE, over 50% of the cases are associated with thrombosis at another site.2, 15 The symptoms of PE are often masked by other conditions in children, including pleuritic pain, dyspnea, coughing, and hemoptysis. The intensity of the symptoms depends upon cardiorespiratory comorbidities and the degree of vascular obstruction. In cases with an obstruction of more than 50%, heart failure and shock occur.32
Thrombosis at uncommon sites (not in limbs) has a prevalence which should not be overlooked in pediatrics.33 The abdominal disease may evolve with acute pain, and hepatosplenomegaly or often remain asymptomatic until the establishment of the clinical portal hypertension.31, 33 Nervous system thrombosis presents symptoms of cranial hypertension, with headaches, vomiting and focal neurological signs.31
Diagnosis
There are to date, validation issues to be resolved regarding the diagnosis of pediatric VTE. The imaging method depends on the location of the thrombosis. Doppler ultrasonography (DUS) is recommended in case of extremity and abdominal thrombosis.34, 35 DUS is not invasive and does not require radiation, however some aspects can complicate the reliability of the interpretation of the exam in children, such as narrow veins, low pulse pressure and presence of a CVC.34 Ultrasonography has a low sensitivity for detecting thrombosis in intrathoracic central vessels, such as the superior vena cava and atrium, and requires complementary exams, such as computerized tomography (CT) or magnetic resonance imaging (MRI).35
DUS is considered the frontline imaging method for hepatic, portal and renal thrombosis.28, 36 In the portal thrombus, an aspect that helps clarify the time installation of the disease is the presence of collaterals that have developed around the obstructed veins and are known as the cavernous transformation. This process begins within 5 weeks of the thrombotic event.36 Computerized tomography angiography (CTA) or MRI are employed to evaluate cerebral thrombosis and inconclusive cases of abdominal VTE.35, 36
For PE investigation, angiotomography and the ventilation/perfusion scintigraphy are indicated. Cardiopulmonary comorbidities may hamper the interpretation of the scintigraphy.32 Despite the disadvantages of the exposure to radiation, the CTA became the preferred method for diagnosis of PE, due to practicality and greater sensitivity. The characteristic finding is the presence of a complete or partial pulmonary arterial filling defect. The echocardiogram can directly visualize thrombi or show evidence of right heart strain, that is indicative of poor evolution. The method is useful in critically ill children, when more invasive testing is not possible.37
The d-dimer negative predictive value has not been validated for children and therefore should not be used to exclude VTE.26 Studies have demonstrated that in up to 40% of the confirmed cases of PE, the d-dimer is negative. Thus, due to unspecific symptoms and lack of an efficient risk score, the best approach for early diagnosis is clinical awareness when the case is a child with a respiratory condition and prothrombotic risk factors.32
Biomarkers, such as cardiac troponin, a brain type natriuretic peptide, have been associated with evolution in adults with PE. However, their clinical utility in pediatrics has not yet been investigated.37
Treatment
VTE is treated with anticoagulants in an attempt to prevent extension and embolization of the thrombus with consequent venous recanalization. Unfractionated heparin (UFH), low molecular weight heparin (LMWH) and oral vitamin K antagonist (VKA) are the major options for anticoagulation therapy.38, 39
Other drugs such as bivalirudin, argatroban and fondaparinux have been used in pediatric patients, however the existing data regarding safety and efficacy is limited, and for this reason will not be presented herein.35 There are new direct oral non-vitamin K anticoagulants available for adults, however these drugs are currently being investigated in phase III clinical trials for pediatric patients.
UFH has the lowest half-life and a specific antidote, which should therefore be indicated in case of a greater risk of bleeding or the need for an invasive procedure, as the effect can be quickly reversed. The drug is also preferred in the setting of renal dysfunction, as the renal elimination is negligible.35, 38, 39
The guidelines on the ideal method for monitoring UFH are not yet clear, therefore both the activated partial thromboplastin time (aPTT) and anti-Xa assays are used in clinical practice. However, levels of aPTT may be difficult to monitor in the presence of a prolonged aPTT baseline, such as might occur due to the physiological factor deficiency during the first year of life.35, 38 The correlation between aPTT and anti-FXa has been demonstrated to be weak, especially in children under 2 years of age.40 Additionally, inflammation may further increase FVIII levels and shorten the aPTT, thereby rendering the method inaccurate for monitoring the heparin effect in neonates and older children. Therefore, the measurement of a heparin specific anti-FXa can assist in monitoring the UFH effect in both these scenarios.35, 40
Heparin-induced thrombocytopenia (HIT) and osteoporosis due to chronic use figure among the side-effects of UFH.35, 38, 39 However HIT is a very rare complication, occurring in up to 2.3% of chidren.35 A systematic review on heparin-induced thrombocytopenia showed that the incidence of HIT in children has been overestimated.41
Despite the LMWH, represented by enoxaparin, being the most commonly used anticoagulant in pediatrics, the medication has not been approved for treatment in this age group. In children, age-dependent weight-based dosing is used and then adjusted by monitoring.35, 38 The benefits of this drug include a lower interaction with other drugs and diet, in addition to a lower rate of osteoporosis and HIT.38, 39
The most utilized VKA worldwide is warfarin, available only in pills. The use is limited due to its interference with various drugs, food, and narrow therapeutic window, requiring constant monitoring. Due to the vitamin K in milk, this drug should not be used for infants who are on a milk diet, and may only be considered for older children under chronic treatment. Due to the slow action onset, warfarin requires a bridge with either UFH or LMWH at the beginning of the treatment.26, 38
Table 2 summarizes the main characteristics of anticoagulants. Complete data regarding posology and adjustment should be sought from specialized guidelines.38
Table 2.
Anticoagulant drugs in pediatric patients.
| Mechanism of action | Half life | Excretion | Therapeutic dose | Monitoring | Antidote | |
|---|---|---|---|---|---|---|
| Unfractionated heparin | Indirect thrombin inhibitor | 1–2 h | Endothelialreticulum system | Loading 75 U/kg bolus IV Maintenance <1 year: 28 U/kg/h >1 year: 20 U/kg/h |
APTT (60–85 s) Anti Xa activity (0.35–0.7 U/ml) |
Protamine sulfate |
| Low molecular weight heparin (enoxaparin) | Xa Factor inhibitor | 3–6 h | Renal |
Preterm neonates 2 mg/kg/dose every 12 h Term neonates 1.7 mg/kg/dose every 12 h SC <2 months: 1.5 mg/kg/dose every 12 h SC >2months: 1 mg/kg/dose every 12 h SC |
Anti-Xa activity (0.5–1.0 U/ml) | Protamine Sulfate (partial reversal) |
| Vitamin K antagonist | Vitamin K inhibitor | 36 hr | Hepatic | Loading 0.6 mg/kg – single dose daily | PT/INR (2–3) | No antidote Vitamin K Prothrombin Complex concentrate to restore coagulation factors |
In catheter-related neonatal thrombosis, the location and extension of the thrombus should be considered. Spontaneous resolution is prone to occur in nonocclusive thrombosis.30 Therefore, in extracardiac thrombi with no occlusion, a doppler ultrasound control to monitor for thrombus progression is sufficient. Indubitably, in the case of no improvement observed during the radiologic and clinical monitoring, the antithrombotic therapy should be initiated.30
On the other hand, in intracardiac thrombi or in other vessel occlusions, heparin should be started immediately. In neonates a higher dose of the drug is required to reach the ideal level of anti-Xa, due to the rapid clearance and physiological reduction of antithrombin.30
In cases associated with cancer, thrombocytopenia, due to the underlying disease or secondary to chemotherapy, represents an aggravating risk for bleeding. However, even in the presence of thrombocytopenia, anticoagulation should be prescribed in therapeutic doses during the acute phase, simultaneously with platelet concentrate, to maintain the platelet level over 50,000/mm3. In VTE secondary to asparaginase, it should be discontinued at diagnosis and resumed after clinical stabilization of the thrombosis.18
Treatment duration depends upon the triggering of the VTE factor. Spontaneous thrombosis is anticoagulated for 6–12 months, whereas events secondary to reversible risk factors (surgery, CVC, trauma) should be treated for three months. When the triggering factor is persistent (neoplasia, nephrotic syndrome, CVC), after three months of anticoagulation, the drug is maintained at a prophylactic dose.26, 38
According to the gold standard guideline in the field, thrombophilia should not influence the anticoagulation duration.38 Nevertheless; there is a tendency to maintain an extended period of treatment in the cases of APS, due to the high risk of recurrence attributed to this condition.26
Thrombolysis is a therapeutic modality that consists in systemic or guided-catheter fibrinolytic infusion, in order to quickly dissolve clots. To reduce the risk of bleeding, laboratory tests should be requested, namely: platelet count, prothrombin time (PT), aPTT, fibrinogen and, in neonates, a head ultrasound to rule out intracranial hemorrhage. The drug of choice is the recombinant tissue plasminogen activator (rt-PA), and this drug is indicated in extreme life-threatening situations, such as PE with shock or extensive large vessel thrombosis of the superior or inferior vena cava, or an intracardiac thrombus.26, 38, 39
Prevention strategies for pediatric VTE
The use of primary thromboprophylaxis has a limited evidence base in children and most indications are in the field of cardiology (Table 3).
Table 3.
Systemic primary thromboprophylaxis in pediatrics.
| Indicationa | Level of evidence |
|---|---|
| Long-term total parenteral nutrition | 2C |
| Hemodialysis | 2C |
| Moderate or giant coronary aneurysms in Kawasaki disease | 2C |
| Cardiopathy | |
| Fontan surgery | 1B |
| Cardiac catheterization via an artery | 1A |
| Bilateral cavopulmonary shunt/Blalock-Taussig shunt | 2C |
| Ventricular assist devices | 2C |
| Dilated cardiomyopathy | 2C |
| Primary Pulmonary Hypertension | 2C |
| Endovascular Stents | 2C |
| Prosthetic heart valvesb | |
Recommendations from the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 9th edition, 2012.
Recommendations from the adult population guidelines.
Regarding thrombophrophylaxis in CVC42 patients, or in those with cancer,43 none of the interventions with antithrombotic agents showed any clear benefit in reducing the incidence of VTE. Therefore, there are no guidelines for VTE prophylaxis in these groups of patients. One exception is the use of a CVC among newborns, which should be flushed via continuous low-dose UFH (0.5 U/kg/h).38 The type and site of the catheter appear to have an influence upon the risk for thrombosis, but an internal CVC and that which is placed in the jugular vein cause lower risks.42
Since the beginning of the XXI Century, the expression “thrombosis epidemic in pediatric hospitals1” has been considered and has had a consequent implementation of preventive measures to hospital-associated VTE (HA-VTE). In spite of some experience with thrombophrophylaxis for hospital diseases at isolated centers,44, 45 there are no multi-institutional validated risk assessment models in the literature.
The Children's Hospital Acquired Thrombosis Database (CHAT) registry is an ongoing multicenter study that aims to develop a risk assessment model. Results from this study will be the basis for randomized clinical trials to evaluate the safety and efficacy of pediatric HA-VTE prevention.46
Challenges of a Brazilian Registry
First, we have to ask why there are no previous multicentric studies on pediatric VTE in Brazil, when compared to other important international publications in the country. We have to consider the territorial extension, the low incidence of the disease, which requires the participation of innumerous centers and multiprofessional teams, including pediatricians, hematologists, radiologists and nurses. Additionally, a qualified laboratory for diagnosis and therapeutic monitoring is required. These limitations have been recently discussed at the 2017 Congress of the Brazilian Hematology Society (ABHH), with the agreement of all participants.
The central issue however, is political intent and strategy fomentation. Health policies must be aware of the problem and direct specific efforts should be made to circumvent the complexity involved, such as what has been seen with “The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism”, a public health program of the United States to reduce the incidence of VTE.47
In view of the above, there are no doubts regarding the benefits of registering the disease in the country. In addition to providing subsidies for strategic policy actions, this registry would also have an educational impact by adjusting current clinical practice to current standard recommendations, such as the Brazilian VTE registry in adults in the 1990s.48
The creation and improvement of educational programs in the field of Pediatric Hemostasis would also represent the backbone for the construction of a system that would be surveying the children with thromboembolic diseases.
Conclusion
According to international data, the rate of childhood VTE has been progressively growing. Once the problem has been clearly established, the aim is to elaborate treatment guidelines and prophylactic measures for this disorder in this age group.
In Brazil, the knowledge and actions taken for this disease remain incipient. The lack of epidemiological data, regardless as to how rudimentary, leads to the impossibility of estimating the size of the problem and how the issue impacts the clinical field and public health. This lack of information impairs the calculation of the costs regarding the disease and consequently, the design of specific policies in the field.
In conclusion, a national pediatric VTE registry is relevant in the perspective of tracing an epidemiological picture of the disease and sensitizing caregivers to strategic policy actions aimed at creating a qualified network of care for these patients.
Conflicts of interest
The authors have no conflicts to declare.
References
- 1.Andrew M., David M., Adams M., Ali K., Anderson R., Barnard D. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251–1257. [PubMed] [Google Scholar]
- 2.Setty B.A., O’Brien S.H., Kerlin B.A. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer. 2012;59(2):258–264. doi: 10.1002/pbc.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White R.H., Dager W.E., Zhou H., Murin S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006;96(3):267–273. doi: 10.1160/TH06-07-0365. [DOI] [PubMed] [Google Scholar]
- 4.Mello T.B., Orsi F.L., Montalvao S.A., Ozelo M.C., de Paula E.V., Annichinno-Bizzachi J.M. Longterm prospective study of recurrent venous thromboembolism in a Hispanic population. Blood Coagul Fibrinolysis. 2010;21(7):660–665. doi: 10.1097/MBC.0b013e32833ceaef. [DOI] [PubMed] [Google Scholar]
- 5.van Ommen C.H., Heijboer H., Büller H.R., Hirasing R.A., Heijmans H.S., Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr. 2001;139(5):676–681. doi: 10.1067/mpd.2001.118192. [DOI] [PubMed] [Google Scholar]
- 6.Gibson B., Chalmers E., Bolton-Maggs P., Henderson D., Lynn R. Thromboembolism in childhood: a prospective two-year BPSU study in the United Kingdom. Br J Haematol. 2004;125(Suppl. 1):1–72. [Google Scholar]
- 7.Schmidt B., Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics. 1995;96(Pt 1):939–943. [PubMed] [Google Scholar]
- 8.Nowak-Göttl U., von Kries R., Göbel U. Neonatal symptomatic thromboembolism in Germany: two year survey. Arch Dis Child Fetal Neonatal Ed. 1997;76(3):F163–F167. doi: 10.1136/fn.76.3.f163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright J.M., Watts R.G. Venous thromboembolism in pediatric patients: epidemiologic data from a pediatric tertiary care center in Alabama. J Pediatr Hematol Oncol. 2011;33(4):261–264. doi: 10.1097/MPH.0b013e3182134111. [DOI] [PubMed] [Google Scholar]
- 10.Raffini L., Huang Y.-S., Witmer C., Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 11.Stein P.D., Kayali F., Olson R.E. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145(4):563–565. doi: 10.1016/j.jpeds.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Kerlin B.A. Current and future management of pediatric venous thromboembolism. Am J Hematol. 2012;87(Suppl. 1):S68–S74. doi: 10.1002/ajh.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuckuviene R., Christensen A.L., Helgestad J., Johnsen S.P., Kristensen S.R. Pediatric venous and arterial noncerebral thromboembolism in Denmark: a nationwide population-based study. J Pediatr. 2011;159(4):663–669. doi: 10.1016/j.jpeds.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 14.Jaffray J., Young G. Developmental hemostasis: clinical implications from the fetus to the adolescent. Pediatr Clin North Am. 2013;60(6):1407–1417. doi: 10.1016/j.pcl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Monagle P., Adams M., Mahoney M., Ali K., Barnard D., Bernstein M. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47(6):763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Stokes S., Breheny P., Radulescu A., Radulescu V.C. Impact of obesity on the risk of venous thromboembolism in an inpatient pediatric population. Pediatr Hematol Oncol. 2014;31(5):475–480. doi: 10.3109/08880018.2014.886315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemoto C.M., Sohi S., Desai K., Bharaj R., Khanna A., McFarland S. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332–338. doi: 10.1016/j.jpeds.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 18.van Ommen C.H., Chan A.K. Supportive care in pediatric cancer: the road to prevention of thrombosis. Semin Thromb Hemost. 2014;40:371–381. doi: 10.1055/s-0034-1370795. [DOI] [PubMed] [Google Scholar]
- 19.Nowak-Göttl U., Junker R., Kreuz W., von Eckardstein A., Kosch A., Nohe N. Risk of recurrent venous thrombosis in children with combined prothrombotic risk factors. Blood. 2001;97(4):858–862. doi: 10.1182/blood.v97.4.858. [DOI] [PubMed] [Google Scholar]
- 20.van Ommen C.H., Heijboer H., van den Dool E.J., Hutten B.A., Peters M. Pediatric venous thromboembolic disease in one single center: congenital prothrombotic disorders and the clinical outcome. J Thromb Haemost. 2003;1(12):2516–2522. doi: 10.1046/j.1538-7836.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 21.Revel-Vilk S., Chan A., Bauman M., Massicotte P. Prothrombotic conditions in an unselected cohort of children with venous thromboembolic disease. J Thromb Haemost. 2003;1(5):915–921. doi: 10.1046/j.1538-7836.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 22.Young G., Albisetti M., Bonduel M., Brandao L., Chan A., Friedrichs F. Impact of inherited thrombophilia on venous thromboembolism in children: a systematic review and meta-analysis of observational studies. Circulation. 2008;118(13):1373–1382. doi: 10.1161/CIRCULATIONAHA.108.789008. [DOI] [PubMed] [Google Scholar]
- 23.Thom K., Male C., Mannhalter C., Quehenberger P., Mlczoch E., Luckner D. No impact of endogenous prothrombotic conditions on the risk of central venous line-related thrombotic events in children: results of the KIDCAT study (KIDs with Catheter Associated Thrombosis) J Thromb Haemost. 2014;12(10):1610–1615. doi: 10.1111/jth.12699. [DOI] [PubMed] [Google Scholar]
- 24.Avcin T., Cimaz R., Silverman E.D., Cervera R., Gattorno M., Garay S. Pediatric antiphospholipid syndrome: clinical and immunologic features of 121 patients in an international registry. Pediatrics. 2008;122(5):e1100–e1107. doi: 10.1542/peds.2008-1209. [DOI] [PubMed] [Google Scholar]
- 25.Raffini L. Thrombophilia in children: who to test, how, when, and why? ASH Educ Program Book. 2008;2008(1):228–235. doi: 10.1182/asheducation-2008.1.228. [DOI] [PubMed] [Google Scholar]
- 26.Chalmers E., Ganesen V., Liesner R., Maroo S., Nokes T., Saunders D. Guideline on the investigation, management and prevention of venous thrombosis in children. Br J Haematol. 2011;154(2):196–207. doi: 10.1111/j.1365-2141.2010.08543.x. [DOI] [PubMed] [Google Scholar]
- 27.Klaassen I.L., van Ommen C.H., Middeldorp S. Manifestations and clinical impact of pediatric inherited thrombophilia. Blood. 2015;125(7):1073–1077. doi: 10.1182/blood-2014-05-536060. [DOI] [PubMed] [Google Scholar]
- 28.Moudgil A. Renal venous thrombosis in neonates. Curr Pediatr Rev. 2014;10(2):101–106. doi: 10.2174/157339631002140513101845. [DOI] [PubMed] [Google Scholar]
- 29.Revel-Vilk S. Central venous line-related thrombosis in children. Acta Haematol. 2006;115(34):201–206. doi: 10.1159/000090936. [DOI] [PubMed] [Google Scholar]
- 30.van Ommen C.H., Sol J.J. Developmental of hemostasis and management of central venous catheter thrombosis in neonates. Semin Thromb Hemost. 2016;42(7):752–759. doi: 10.1055/s-0036-1592299. [DOI] [PubMed] [Google Scholar]
- 31.Young G. Diagnosis and treatment of thrombosis in children: general principles. Pediatr Blood Cancer. 2006;46(5):540–546. doi: 10.1002/pbc.20626. [DOI] [PubMed] [Google Scholar]
- 32.Dijk F.N., Curtin J., Lord D., Fitzgerald D.A. Pulmonary embolism in children. Paediatr Respir Rev. 2012;13(2):112–122. doi: 10.1016/j.prrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Pergantou H., Avgeri M., Komitopoulou A., Xafaki P., Kapsimali Z., Mazarakis M. Venous thromboembolism at uncommon sites in neonates and children. J Pediatr Hematol Oncol. 2014;36(8):624–629. doi: 10.1097/MPH.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 34.Spentzouris G., Scriven R.J., Lee T.K., Labropoulos N. Pediatric venous thromboembolism in relation to adults. J Vasc Surg. 2012;55(6):1785–1793. doi: 10.1016/j.jvs.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 35.Chan A.K., Monagle P. Updates in thrombosis in pediatrics: where are we after 20 years? Hematology Am Soc Hematol Educ Program. 2012;2012:439–443. doi: 10.1182/asheducation-2012.1.439. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R., Kerlin B.A. Thrombosis of the abdominal veins in childhood. Front Pediatr. 2017;5:188. doi: 10.3389/fped.2017.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaidi A.U., Hutchins K.K., Rajpurkar M. Pulmonary embolism in children. Front Pediatr. 2017;5:170. doi: 10.3389/fped.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monagle P., Chan A.K., Goldenberg N.A., Ichord R.N., Journeycake J.M., Nowak-Göttl U. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9HTed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl.) doi: 10.1378/chest.11-2308. e737S–801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goel R., Vedantham S., Goldenberg N.A. Antithrombotic therapies: anticoagulation and thrombolysis. Pediatr Clin North Am. 2013;60(6):1463–1474. doi: 10.1016/j.pcl.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Chan A.K., Black L., Ing G., Brandão L.R., Williams S. Utility of aPTT in monitoring unfractionated heparin in children. Thromb Res. 2008;122(1):135–136. doi: 10.1016/j.thromres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Avila M.L., Shah V., Brandão L.R. Systematic review on heparin-induced thrombocytopenia in children: a call to action. J Thromb Haemost. 2013;11:660–669. doi: 10.1111/jth.12153. [DOI] [PubMed] [Google Scholar]
- 42.Biss T.T. Venous thromboembolism in children: is it preventable? Semin Thromb Hemost. 2016;42(6):603–611. doi: 10.1055/s-0036-1581100. [DOI] [PubMed] [Google Scholar]
- 43.Schoot R.A., Kremer L.C., van de Wetwering M.D., van Ommen C.H. Systemic treatment for the prevention of venous thrombo-embolic events in pediatric cancer patients with tunnelled central venous catheters. Cochrane Database Syst Rev. 2013;11(9):CD009160. doi: 10.1002/14651858.CD009160.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Atchison C.M., Arlikar S., Amankwah E., Ayala I., Barrett L., Branchford B.R. Development of a new risk score for hospital-associated venous thromboembolism in noncritically ill children: findings from a large single-institutional case-control study. J Pediatr. 2014;165(4):793–798. doi: 10.1016/j.jpeds.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Development of a pediatric-specific clinical probability tool for diagnosis of venous thromboembolism: a feasibility study. Pediatr Res. 2015;77(3):463–471. doi: 10.1038/pr.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaffray J., Mahajerin A., Young G., Goldenberg N., Ji L., Sposto R. Multi-institutional registry of pediatric hospital-acquired thrombosis cases: The Children's Hospital-Acquired Thrombosis (CHAT) project. Thromb Res. 2018;161:67–72. doi: 10.1016/j.thromres.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Office of the Surgeon General (US), National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism [Internet]. Rockville (MD): Office of the Surgeon General (US); 2008. Available from: http://www.ncbi.nlm.nih.gov/books/NBK44178/ [cited 18.07.18]. [PubMed]
- 48.Caiafa J.S., de Bastos M., Moura L.K., Raymundo S. Managing venous thromboembolism in Latin American patients: emerging results from the Brazilian registry. Semin Thromb Hemost. 2002;28(3):47–50. doi: 10.1055/s-2002-34076. [DOI] [PubMed] [Google Scholar]
- 49.Heit J.A., Spencer F.A., White R.H. The epidemiology of venous thrombosis. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozaki A, Bartholomew JR. Venous thromboembolism (deep venous thrombosis & pulmonary embolism) [Internet]. Cleveland Clinic Foundation. Center for Continuing Education 2112. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/venous-thromboembolism/#top [cited 10.08.18].