Fig. 7.
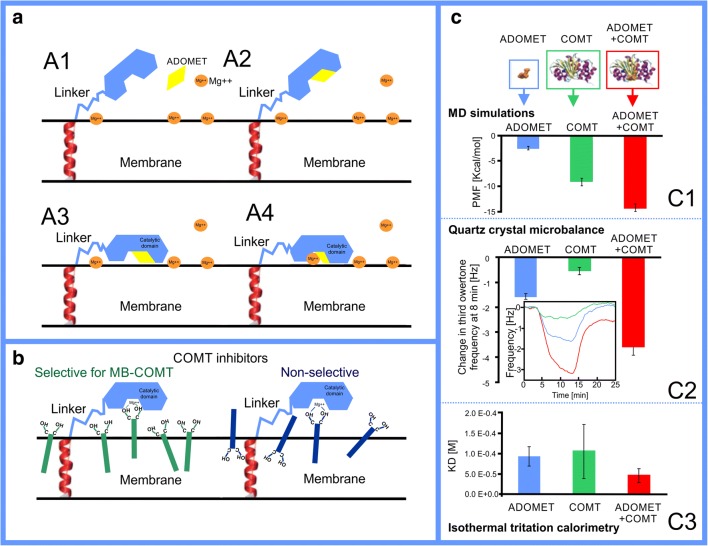
The pivotal role of the membrane in membrane-bound catechol-o-methyl transferase catalysis and selective inhibition. a Steps of catalytic mechanism of the membrane-bound catechol-o-methyl transferase (MB-COMT): (A1) the catalytic domain interacts weakly with membrane in the apo form; (A2) the cofactor S-adenosyl-l-methionine (ADOMET) binds to the catalytic site of MB-COMT; (A3) MB-COMT in complex with ADOMET opens the catalytic site towards the membrane, which, in turn, allows the protein to bind to the membrane surface; (A4), finally, the MB-COMT binds an Mg2+ ion that is already present at the membrane surface. b The behavior of MB-COMT selective vs. non-selective inhibitors in the membrane: selective inhibitors orient catechol group towards the water phase and, in contrast, non-selective inhibitors could be oriented less optimally in relation to the MB-COMT catalytic site. c The estimations of interactions of the ADOMET and catalytic domain of COMT in complex and separately with lipids indicate that the catalytic domain is preferably membrane-oriented: (C1) the free energy changes when protein is pulled away from the lipid bilayer; (C2) quartz crystal microbalance (QCM) frequency changes during interaction with the lipid bilayer; (C3) dissociation constant (inverse of affinity) from lipid bilayer (vesicle) determined by isothermal calorimetry. Reproduced with permission from ref. [146]. Copyright 2018 the Royal Society of Chemistry