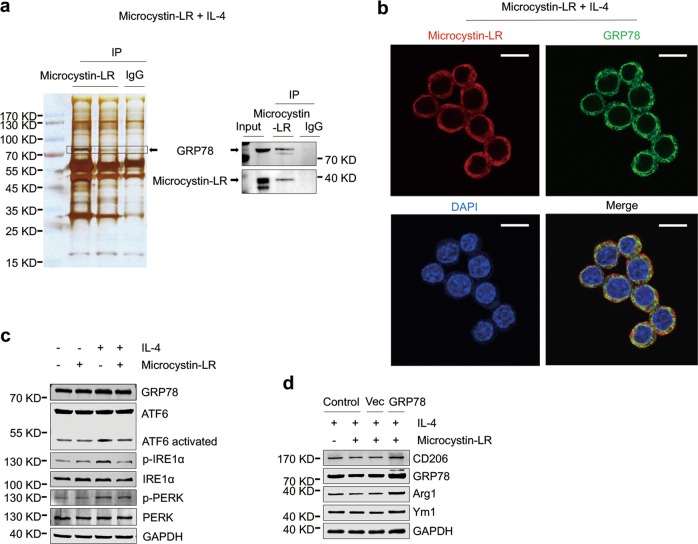
Fig. 6. Microcystin-LR inhibits the responses to endoplasmic reticulum stress by interaction with GRP78.
RAW264.7 cells were treated as explained in Fig. 5. a Total protein extracts from RAW264.7 cells pretreated with IL-4 and microcystin-LR were immunoprecipitated with two independent microcystin-LR antibodies (left lane: MOB-647, Creative Biolabs; middle lane: ALX-804-320, Alexis Biochemicals). A ~ 78 kDa protein band was subsequently identified as glucose-regulated protein 78 kDa (GRP78) using mass spectrometry (left panel) and western blot (right panel). b Subcellular localization of microcystin-LR (red) and GRP78 (green) was examined using immunofluorescence staining. DAPI was used for nuclear staining (blue). Scale bar: 50 μm. c Cellular protein expression of GRP78 and its three transmembrane sensors including activating transcription factor 6 (ATF6), inositol requiring enzyme 1α (IRE1α) and PKR-like endoplasmic reticulum kinase (PERK), together with their activated forms as indicated was measured by western blot. d GRP78 rescue assay was performed by transfecting RAW264.7 cells with the recombinant GRP78 expression plasmid (pcDNA3-GRP78). Control cells without transfection, vector (pcDNA3.1) and the pcDNA3-GRP78 transfected cells were treated as indicated for 48 h. The expressions of M2 macrophage markers, CD206, Arg1 and Ym1, and GRP78 were analyzed by western blot.