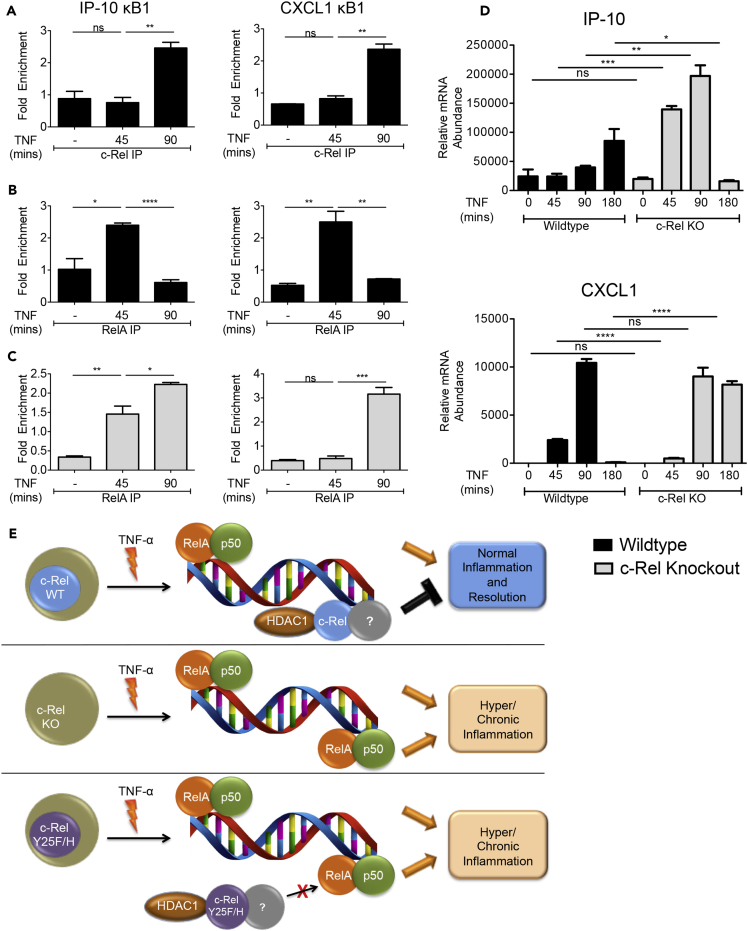
Figure 7.
c-Rel Represses RelA DNA Binding and Transactivation In Vivo
(A–D) Wild-type or c-Rel knockout mice (n = 3) were injected with PBS or TNF-α (5 μg/mouse). Mice were euthanized at 45, 90, or 180 min after injection, and single-cell suspension of liver was processed for chromatin immunoprecipitation and qPCR. ChIP was performed using control IgG and either c-Rel (A) or RelA (B and C) antibodies. Enrichment of IP-10 and CXCL1 promoter regions in the ChIP samples were examined by qPCR in triplicate. (D) TNF-α-induced in vivo expression of IP-10 (top) and CXCL1 (bottom) relative to that of ribosomal protein L32 in the liver was examined by qPCR in triplicate. Data are presented as mean ± standard error of mean (SEM). p Values were obtained by unpaired Student’s t test; ****p < 0.0001, ***p < 0.001, **p < 0.01, ns non-significant.
(E) Hypothetical schematic model describing DNA-binding-dependent suppression of RelA-dependent transcription by c-Rel. Top. c-Rel-containing dimers with co-repressor, HDAC1, occupy certain RelA-binding sites limiting RelA-induced gene expression. Middle. Absence of c-Rel exposes sites repressed by c-Rel for RelA binding and enhanced inflammatory gene expression. Bottom. Mutation of Y25 in c-Rel blocks c-Rel's DNA binding allowing enhanced RelA binding and inflammatory gene expression.