Abstract
Aging is characterized by dynamic changes at metabolic level that lead to modifications in the composition of the metabolome. Since the identification of biomarkers that can discriminate people of different age and health status has recently attracted a great interest, we wondered whether age-specific changes in the metabolome could be identified and serve as new and informative biomarkers of aging and longevity. In the last few years, a specific branch of metabonomics devoted to the study of volatile organic compounds (VOCs) has been developed. To date, little is known about the profile of specific VOCs in healthy aging and longevity in humans; therefore, we investigated the profile of VOCs in both urine and feces samples from 73 volunteers of different age including centenarians that represent useful “super-controls” to identify potential biomarkers of successful aging and footprints of longevity. To this purpose, we performed a discriminant analysis by which we were able to identify specific profiles of urinary and fecal VOCs. Such profiles can discriminate different age groups, from young to centenarians, and, even more interesting, centenarians’ offspring from age-matched controls. Moreover, we were able to identify VOCs that are specific for the couples “centenarians – offspring” or the trios “centenarians – offspring – spouse,” suggesting the possible existence of a familiar component also for VOCs profile.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00143-6) contains supplementary material, which is available to authorized users.
Keywords: Centenarians, Volatile organic compounds, Human longevity, Biomarkers
Introduction
Aging is a complex phenomenon characterized by a gradual accumulation of damages at cellular level that leads to a progressive deterioration of the normal biological functions and an increased susceptibility to diseases. In the last years, a growing number of studies have been devoted to unravelling the cellular and molecular mechanisms underpinning human aging. In this framework, the identification of biomarkers of aging has recently attracted great interest. Ideally, these biomarkers should be able to discriminate not only persons of different age but also people with different health state and/or rate of aging. Accordingly, higher or lower levels of specific biomarkers may indicate an “accelerated” rate of aging, forecasting unhealthy aging and elevated susceptibility to diseases, or, on the contrary, a “decelerated” rate, which should be in principle associated with healthy aging and longevity (Franceschi et al. 2018, 2019).
Human longevity is the result of the interactions of several factors (genetic predispositions, epigenetic setting up, environmental conditions) that allow an individual to achieve the extreme limits of lifespan and escape, postpone, or survive the main age-related diseases. In this scenario, centenarians represent the best example of successful aging and longevity and can be considered as a sort of “super-controls” useful when studying the mechanisms of aging and of the major age-related diseases (Giuliani et al. 2017).
In recent years, a number of potential biomarkers of age have been proposed, which can contribute to identifying subjects at higher risk of developing age-associated diseases (Franceschi et al. 2018). These include as an example epigenetic clocks (Horvath 2013; Hannum et al. 2013; Bacalini et al. 2019) and the ratio of specific N-glycans on circulating proteins (Vanhooren et al. 2010; Borelli et al. 2015). In this context, metabonomics is considered a useful approach to identify potential biomarkers of the aging process and footprints of longevity (Collino et al. 2013; Montoliu et al. 2014). In the last few years, a specific branch of metabonomics devoted to the study of volatile organic compounds (VOCs) has been developed. Actually, there is a growing interest for VOCs, as they are considered non-invasive diagnostic biomarkers (Probert et al. 2009; Ahmed et al. 2016; Liu et al. 2018). VOCs are low-weight carbon-based molecules involved in different physiological processes and can originate either from the human body (endogenous VOCs), reflecting the metabolic conditions of the individual, or from external sources, mainly food and drugs (exogenous VOCs). Accordingly, VOCs profile may vary with age, gender, diet, physiological and nutritional status, and habits (alcohol consumption or smoking); therefore, VOCs could be considered as the “odour-fingerprint” of individuals (Shirasu and Touhara 2011). The (patho)physiological conditions of the individuals create specific spectra of VOCs, detectable in sweat, exhaled breath, blood, urine, and feces (Amann et al. 2014; Filipiak et al. 2016), and an increasing number of studies indicate that specific VOCs profiles are disease-associated and can thus represent a possible tool to identify patients and/or monitor disease progression as they are strongly dependent on metabolism, inflammation, and infections. In particular, the alteration of specific biochemical mechanisms related to oxidative stress, cytochrome p450, carbohydrate, and lipid metabolism yields a VOCs profile that mirrors diseases of kidney, liver, and gastrointestinal tract, as well as neurodegenerative diseases and cancers (Lee et al. 2009; Broza et al. 2014; von Grafenstein et al. 2014).
To date, little (or almost nothing) is known about the profile of specific VOCs in healthy aging and longevity in humans. Some years ago, Mazzatenta et al. (2015) analyzed the VOCs from exhaled breath in healthy centenarians, elderly controls, and young subjects, and they found distinct VOCs patterns in centenarians, suggesting that VOCs from exhaled breath may represent biomarkers of aging and longevity (Mazzatenta et al. 2015). We wondered whether a modification of VOCs profile was occurring with age also in other biological samples, i.e., feces and urines, and whether, also in this case, centenarians would display a peculiar VOCs pattern that could represent a sort of “smell of longevity.” In order to answer these questions, we quantified VOCs in feces and urine samples from 73 volunteers, from 24 to 106 years of age, including centenarians’ offspring, as they represent an additional model of healthy aging (Bucci et al. 2016). The analysis in urine and feces has several advantages over other body matrices. First, the collection of urine and feces is non-invasive and allows for multiple sampling; it does not have quantitative limits and it is practically inexpensive. Moreover, VOCs from these matrices are mostly of endogenous origin, reflecting the individual metabolism rather than the environment where people live.
Our results indicate the existence of specific patterns of urinary and fecal VOCs that can discriminate subjects of different age, from young to centenarians, and, even more interesting, centenarians’ offspring from age-matched controls.
Methods
Subjects and sampling
A total of 73 volunteers of Caucasian ancestry in the age range from 24 to 106 years were recruited and divided into four groups: young subjects (Y), elderly controls (EC), elderly centenarians’ offspring (ECO), and centenarians (CENT), see Table 1. In the ECO group, 10 subjects were offspring of the enrolled centenarians. In the EC group, 5 subjects were spouse of the enrolled centenarians’ offspring. All subjects were enrolled within the framework of the Italian National Project “PRIN 09” (Bologna Unit). The study protocols were approved by the Ethical Committee of Sant’Orsola-Malpighi University Hospital, Bologna, Italy (Ethical clearance EM 157/2011/U issued on Nov. 25, 2011). All subjects signed an informed consent before entering the study. Exclusion criteria were the presence of malignant neoplasia and/or a current therapy with immune suppressor drugs (like cyclosporine, methotrexate, and glucocorticoids) or anticoagulant drugs. A standard questionnaire was administered to the volunteers by nurse staff in order to collect lifestyle data, health status, clinical anamnesis, and details on medications. As for CENT, in the case that the subject was unable to respond autonomously because of hearing or sight problems, the interview was performed with a relative or a caregiver. Y subjects were free of clinically evident diseases, while, as expected, ECO resulted healthier than EC (0.6 and 1.8 comorbidities, respectively), these latter being representative of the general elderly population (Table 1). The health status of CENT was more heterogeneous: while the majority was in good health, some of them suffered from multiple age-related diseases. However, the age of onset of such diseases is very advanced, supporting the idea that centenarians are a model of successful aging (see Supplementary Table 1). The first morning urine samples and feces were collected in sterile tubes and stored at − 80 °C. No difference was found when VOCs analysis was performed by gender (results not showed); therefore, males and females were pooled together.
Table 1.
Experimental sample description
| Group (N) |
Age range |
Sex (N) |
Average number of diseases | Current diseases (N) |
|---|---|---|---|---|
| Y (15) | 24–36 | 4M, 11F | 0 | – |
| EC (20) | 61–85 | 12M, 8F | 1.8 | Arthrosis (7), chronic obstructive pulmonary disease (3), chronic renal failure (1), diabetes (2), hypercholesterolemia (5), hypertension (10), hypothyroidism (2), osteoporosis (4) |
| ECO (16) | 67–76 | 6M, 10F | 0.6 | Arthrosis (2), hypercholesterolemia (2), hypothyroidism (2), osteoporosis (4) |
| CENT (22) | 100–106 | 4M, 18F | 3.1 | Angina pectoris (2), autoimmune diseases (1), cancer (in remission) (1), chronic obstructive pulmonary disease (4), chronic renal failure (3), dementia (2), depression/anxiety (3), diabetes (3), heart failure (5), hepatitis (1), hypertension (14), hyperuricemia (2), hypothyroidism (2), irregular heart rhythm (6), osteoporosis (6) |
Y young, EC elderly controls, ECO elderly centenarians’ offspring, CENT centenarians
Urine and feces sample preparation and SPME-GC/MS analysis
The VOCs were determined by a solid phase microextraction-gas chromatography-mass spectrometry technique (SPME-GC/MS) according to Silva et al. (2011) and Garner et al. (2007) for urine and feces, respectively, with some modification. Samples (200 μg of urines or feces) were put into a 20-mL glass vial, closed with an aluminum cap equipped with a PTFE-septum. The VOC mixture was collected using a stable Flex SPME fiber (75 μm; 2 cm long, CAR/PDMS) (Supelco, Bellefonte, PA, USA) that represents the best fiber for feces and urine, as demonstrated by Dixon et al. (2011). The SPME fiber, conditioned for 30 min at 270 °C in the GC injector in order to eliminate residues, was exposed to headspace for 45 min at 45 °C and 20 min at 40 °C for urines and feces respectively. VOCs were determined by means of a single quadrupole GC/MS apparatus (TRACE GC/MS, Thermo- Finnigan, Waltham, MA, USA). The injector was set at 250 °C. For the first 3 min, the split was set in splitless mode, and the fiber was kept in the injector for 30 min. The GC apparatus was coupled with a Varian CP-WAX-52 capillary column (60 m × 0.32 mm; coating thickness 0.5 μm) and the conditions of the GC program temperature were set according to Povolo et al. (2007): oven temperature was held at 40 °C for 8 min, programmed to 220 °C at a rate of 4 °C/min, and held at 220 °C for 20 min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The transfer-line and the ion source were set at 250 °C. The filament emission current was 70 eV. A mass range from 35 to 270 mz was scanned at a rate of 1.6 amu/s. The acquisition was carried out by electron impact, using the full scan (TIC) mode.
The VOCs were identified by comparison with the mass spectra of the Wiley library (version 2.0-11/2008), by the injection of authentic standards (Sigma–Aldrich), and by calculating the LRI and matching them with bibliographical indexes (Povolo et al. 2007; Serra et al. 2014). Data were expressed as peak percentage of total VOCs.
Statistical analyses
Data of VOCs classes level were evaluated with the following linear model, using the software JMP (SAS, 2007):
where yij = percentage of VOCs classes, μ = mean, AGEi = fixed effect of the ith age factor (Y, CENT, ECO, EC), Sj = random effect of the jth subject (73 levels), and εij = casual error.
Data were retained significant at P < 0.05. Statistical differences between groups were assessed using Tukey’s test, significance being determined at P < 0.05.
VOCs of feces and urine were involved in the multivariate discriminant procedures. Three complementary techniques were used to discriminate the 4 groups (Y, CENT, ECO, and EC): canonical discriminant analysis (CDA), stepwise discriminant analysis (SDA), and discriminant analysis (DA). The CDA is a dimension-reduction technique, related to principal component analysis and canonical correlation, able to perform both univariate and multivariate 1-way analyses. Given a classification character and several interval variables, CDA derives a set of new variables, called canonical functions (CAN), which are linear combinations of the original interval variables, as reported in the following equation:
where di are the canonical coefficients (CC) that indicate the contribution of each variable in composing the CAN, and X is the scores of the n original variables.
As principal components summarize the total variation in the data, CAN summarizes the between-groups variation, highlighting their differences. In general, if k groups are involved in the study, k − 1 CANs are extracted. The effective separation between groups was assessed by using the Mahalanobis distance and the corresponding Hotelling’s T-square test (De Maesschalck et al. 2000). Briefly, the Mahalanobis distance considers the variable covariances in calculating distances between individuals in a multivariate vector space. The ordinary Euclidean distance is a special case of the Mahalanobis distance when variables have equal variances and zero covariances. The Hotelling’s T-square test is an extension of the Student’s t test to the multivariate domain (Mardia 1975).
The minimum number of VOCs able to discriminate the 4 groups was obtained by using the SDA, a statistical technique specifically conceived to select the subset of variables that better separate groups. The SDA was applied to the 110 and 32 VOCs of feces and urine, respectively. The most discriminant VOCs were selected and used for CDA and DA.
The ability of CAN to assign each subject to the 4 groups was calculated as the percent of correct assignment using the DA (Mardia et al. 2000). In practice, the CAN is applied to each subject, thus obtaining a value called discriminant score. Then, the centroids of the 4 groups are calculated and, for each individual, distances from the 4 centroids are evaluated. Each subject is assigned to the 4 groups based on the lowest distance from the 4 group centroids (Mardia et al. 2000).
In order to identify endogenous VOCs produced by metabolism and that are under genetic control, we applied the same multivariate discriminant analysis also on the 10 couples centenarian–their offspring.
Results
Age distribution of fecal VOCs
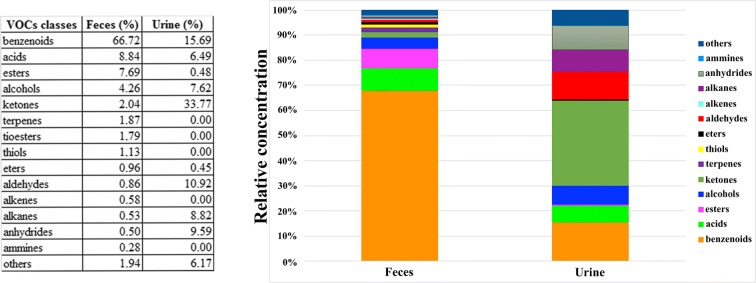
A total number of 110 VOCs were detected in fecal samples (Supplementary Table 2). These VOCs were classified according to their corresponding chemical class and the relative distributions and were included in the following 14 groups: acids, alcohols, aldehydes, alkanes, alkenes, ammines, anhydrides, benzenoids, esters, ethers, ketones, terpenes, thiols, thioethers. The largest portion of VOCs present in feces is represented by benzenoids (66.72%), followed by acids (8.84%), esters (7.69%), and alcohols (4.26%). The smallest portion is represented by alkanes (0.53%), anhydrides (0.50%), and ammines (0.28%) (Fig. 1, left column). In addition, nine compounds belonging to different and less representative classes were grouped as “others.”
Fig. 1.
The relative numbers of the different compounds within each class that have been detected in feces and urine
The analysis has been then conducted by dividing the subjects participating to the study into 4 age groups: centenarians (CENT), elderly centenarians’ offspring (ECO), elderly controls (EC), and young (Y) (see the “Methods” section). Some VOCs were present in all the subjects of each group (100% of occurrence); however, there were VOCs that resulted undetectable (0% of occurrence) in one or two groups (Fig. 2a). For example, as described in Supplementary Table 2, propanoic acid propyl ester and acetic acid butyl ester were undetectable in CENT, while propanoic acid butyl ester was undetectable in CENT and ECO groups. Conversely, 4-methyl-2-pentyl acetate was undetectable in EC, while 10-undecenoic acid methyl ester was undetectable in Y and EC groups. These VOCs belong to ester class and most of them are linked to lipid metabolism.
Fig. 2.
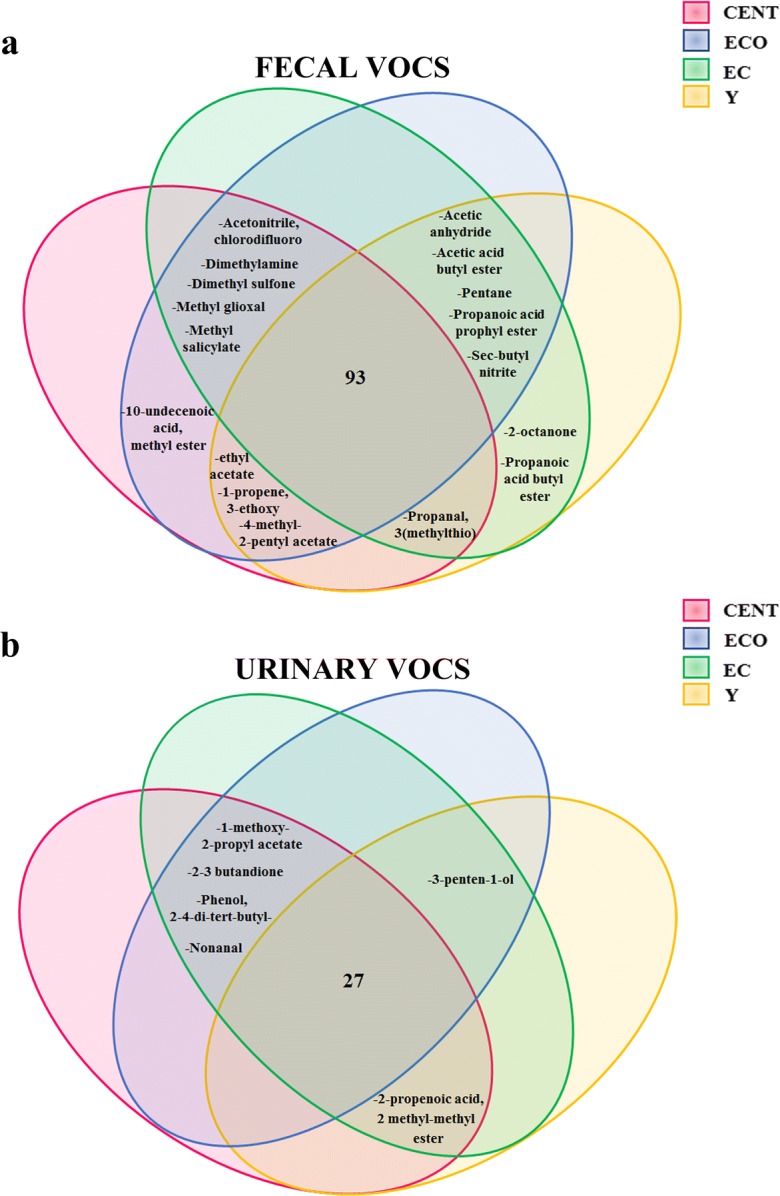
Venn diagrams providing a graphical representation of VOCs from feces and urine. a Number and type of VOCs identified in feces of the four age groups: 93 VOCs are expressed in all age group; the VOCs showed in the diagram are those that result differentially detectable in each group. b Number and type of VOCs identified in urine of the four age groups: 27 VOCs are expressed in all age group; the VOCs showed in the diagram are those that result differentially detectable in each group
Age distribution of urinary VOCs
A total number of 33 VOCs was identified in urine samples (Supplementary Table 3). Also in this case, VOCs were classified according to their corresponding chemical class and the relative distributions and were included in the following 9 groups: acids, alcohols, aldehydes, alkanes, anhydrides, benzenoids, esters, ethers, ketones. Similarly to fecal VOCs, also in this case, there were four less representative compounds that were grouped as “others.” The largest portion of VOCs present in urine is represented by ketones (33.77%), followed by benzenoids (15.69%), aldehydes (10.92%), and anhydrides (9.59%). The smallest portion is represented by esters (0.48%) and ethers (0.45%) (Fig. 1, right column). Most VOCs were present in all the subjects of each group (100% of occurrence); however, as for feces, there were VOCs that resulted undetectable in one group (Fig. 2b). For example, as described in Supplementary Table 3, some VOCs, such as nonanal (aldehydes), 2-3 butandione (ketones), 1-methoxy-2-propyl acetate (ethers), and phenol-2-4-di-tert-butyl- (benzenoids), were undetectable in Y group, while 3-penten-1-ol was undetectable in CENT.
Differences in VOCs abundance in feces and urine from subjects of different age
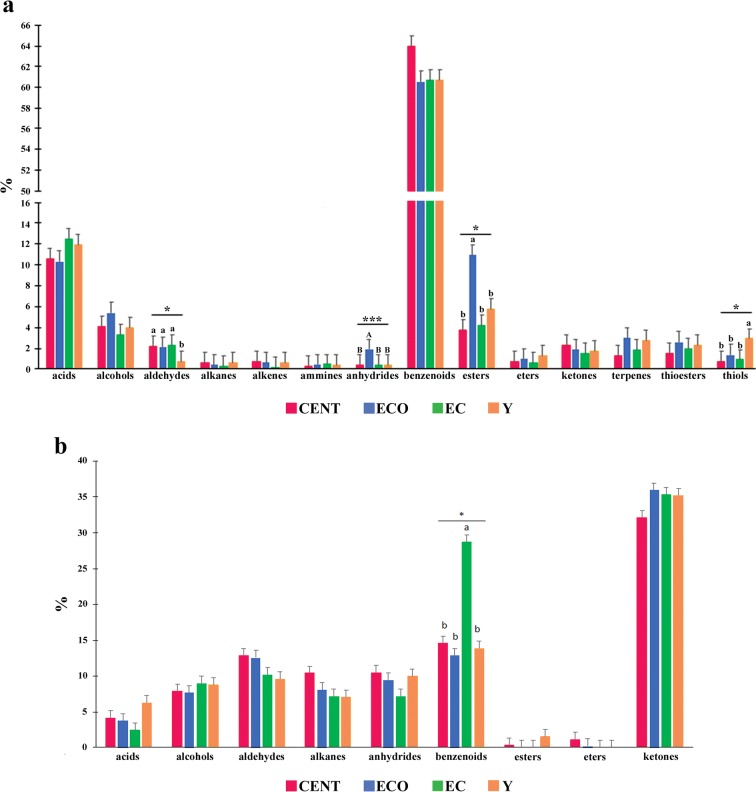
To better understand if there are changes with age in VOCs abundance, we compared the different age groups as far as the relative concentration values of the identified classes of VOCs (Fig. 3a, b).
Fig. 3.
Relative concentration of the class of VOCs detected in feces (a) and urine (b) in each age group. The different colors in histogram correspond to the different groups (CENT, centenarians; ECO, elderly centenarian’s offspring; EC, elderly controls; Y, young subjects)
In feces, the classes of aldehydes, anhydrides, esters, and thiols present significant differences in some groups. Aldehydes and thiols were significantly different in Y group compared with the elderly groups (CENT, ECO, and EC); in particular, Y group showed a lower and higher level for aldehydes and thiols, respectively. In addition, anhydrides and esters classes were significantly higher in ECO group compared with CENT, EC, and Y groups (Fig. 3a).
In urines, the class of benzenoids was significantly higher in EC compared with the other groups. For the other classes, many trends can be observed, although with no statistically significant values (Fig. 3b).
VOCs signature of aging and longevity
To further investigate whether there is a VOCs signature that discriminates age groups, we performed a multivariate discriminant analysis. As far as feces, CDA was applied to the 47 selected VOCs identified (Table 2), which gave three significant (P < 0.01) canonical variables, CAN_1, CAN_2, and CAN_3. The scatter plot reported in Fig. 4 demonstrates the complete segregation of the four age groups (CENT, ECO, EC, and Y). The CAN_1 variable markedly separated EC group from ECO and CENT groups. The original variables mostly involved in this discrimination were formic acid butyl ester, 4-carene, and 1H-indole, 5 methyl (Table 2). The CAN_2 variable separated CENT from ECO and the original variables mostly involved were butanoic acid methyl ester, ethanol, butanoic acid ethyl ester, and butanoic acid, 3 methyl-, 2-methyl propyl ester. Finally, CAN_3 separated Y from the three groups of elders (CENT, ECO, EC) and the original variables mostly involved were dimethyl trisulfide and 1H-indole, 3 methyl.
Table 2.
Canonical coefficients obtained from discriminant analysis in stepwise methods used to classify the most significant VOCs of feces detected in centenarians, elderly centenarians’ offspring, elderly controls, and young
| VOCs | CAN_1 | CAN_2 | CAN_3 |
|---|---|---|---|
| Carbon dioxide | 0.01 | 0.07 | − 0.26 |
| Acetaldehyde | 0.21 | 0.04 | − 0.15 |
| Propanal, 2-methyl- | 0.20 | 0.01 | − 0.30 |
| 2-Butanone | 0.07 | − 0.21 | − 0.15 |
| Cyclopentanol | 0.18 | 0.01 | − 0.32 |
| Acetic acid ethenyl ester | − 0.01 | − 0.02 | 0.01 |
| 2-Pentanone | − 0.08 | − 0.10 | − 0.15 |
| 2-Butanol | − 0.13 | 0.28 | − 0.34 |
| Disulfide dimethyl | 0.06 | 0.24 | 0.05 |
| 2-Hexanone | 0.28 | 0.09 | − 0.11 |
| 1-Butanol | 0.09 | − 0.13 | 0.14 |
| Formic acid, butyl ester | 0.44 | 0.03 | 0.01 |
| 1-Butanamine | − 0.03 | 0.13 | 0.15 |
| Cyclohexene, 1-methyl-4-(1-methylethylidene) | − 0.02 | 0.30 | 0.14 |
| 1-Pentanol | 0.05 | 0.33 | 0.05 |
| Propane 2-ethoxy | − 0.33 | 0.03 | − 0.10 |
| 5-Heptene-2-one-6-methyl | − 0.29 | 0.06 | − 0.15 |
| Dimethyl trisulfide | 0.30 | 0.19 | 0.40 |
| p-Penthylacetophenone | − 0.21 | 0.18 | − 0.20 |
| Acetic acid | 0.16 | 0.04 | 0.13 |
| Propanoic acid | 0.24 | 0.08 | 0.07 |
| Benzaldehyde | − 0.08 | − 0.23 | − 0.23 |
| Propanoic acid 2-methyl | 0.23 | − 0.10 | 0.27 |
| Caproic acid | − 0.04 | − 0.18 | − 0.10 |
| 9-Hexadecen-1-ol, (Z)- | − 0.24 | − 0.08 | − 0.02 |
| Phenilethyl alcohol | − 0.15 | − 0.31 | − 0.10 |
| Indole | − 0.21 | 0.12 | − 0.06 |
| Phosphonic acid (p-hydroxyphenyl) | − 0.32 | 0.05 | − 0.01 |
| 1H-indole, 3 methyl | 0.24 | − 0.21 | − 0.40 |
| Phenol, 3-methyl | 0.28 | − 0.22 | − 0.12 |
| Butane | 0.17 | − 0.26 | − 0.07 |
| Butanoic acid | 0.28 | 0.06 | − 0.05 |
| 1-Butanol 3 methyl | 0.10 | 0.01 | − 0.06 |
| Methanetiol | − 0.15 | 0.07 | 0.17 |
| Benzene-1-ethyl-2-4 dimethyl | − 0.02 | 0.25 | 0.34 |
| Butanoic acid methyl ester | − 0.07 | 0.58 | − 0.22 |
| 4-Carene | 0.46 | 0.34 | − 0.04 |
| Dimethylsulfide | 0.17 | 0.18 | − 0.02 |
| 2-Propanone | − 0.14 | − 0.10 | − 0.07 |
| Butanoic acid 3 methyl | 0.18 | − 0.10 | 0.01 |
| 1H-indole, 5 methyl | 0.47 | − 0.05 | − 0.30 |
| Ethanol | 0.07 | 0.43 | − 0.30 |
| Butanoic acid ethyl ester | − 0.05 | 0.47 | − 0.18 |
| 4-Penten, 2-olo | 0.14 | 0.04 | − 0.10 |
| Propanoic acid ethyl ester | 0.27 | − 0.02 | − 0.01 |
| Butanoic acid propyl ester | 0.09 | − 0.29 | − 0.13 |
| Butanoic acid, 3 methyl-, 2-methyl propyl ester | 0.02 | 0.45 | 0.09 |
| Variance explained (%) | 56.19 | 29.30 | 14.51 |
| Cumulative variance (%) | 56.19 | 85.49 | 100.00 |
VOCs with a higher correlation coefficient are shown in italics
Fig. 4.
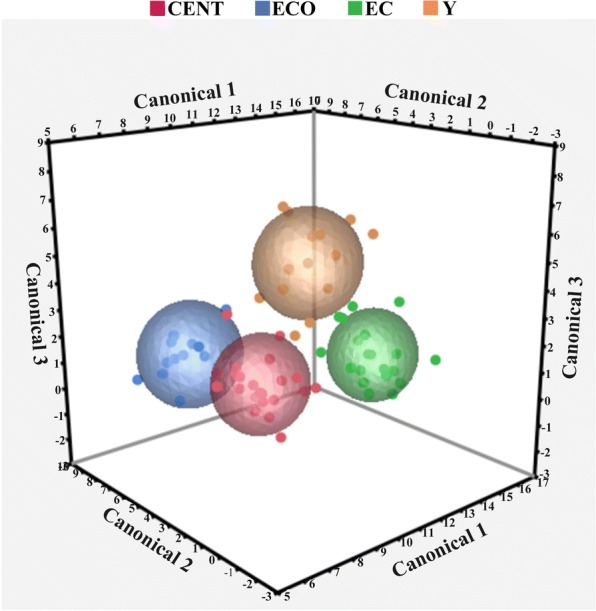
Three-dimensional scatter plot by using canonical discriminant analysis showing the differentiation in VOCs profile from feces of the four age groups: centenarians (CENT), centenarian’s offspring (ECO), elderly controls (EC), young subjects (Y). Canonical_1 variable separated EC group from ECO and CENT groups. Canonical_2 variable separated CENT from ECO. Canonical_3 separated Y from the three groups of elders (CENT, ECO, EC)
As far as urine samples, SDA selected all 33 VOCs identified by the CDA (Table 3), which also gave three significant canonical variables (P < 0.01). The first two variables accounted for 96% of the total variability (Table 3), thus indicating that the multivariate structure of the VOCs could be well represented by only the first two canonical variables. This indication is confirmed by the scatter plot of CAN_1×CAN_2 (Fig. 5), which shows the segregation of the four groups. The CAN_1 variable markedly separated the Y subjects from the other groups. The VOCs mostly involved in this discrimination were 2-butanone, isopropyl alcohol, 2-pentanone, 3-penten-1-ol, and pyrrole (Table 3). CAN_2 separated CENT and ECO from EC, and the VOCs mostly involved were carbon dioxide, benzaldehyde 2,3,6-trichloro, acetone, trichloromethane, benzaldehyde, and butanoic acid (Table 3).
Table 3.
Canonical coefficients obtained from discriminant analysis in stepwise methods used to classify the most significant VOCs of urine detected in centenarians, elderly centenarians’ offspring, elderly controls, and young
| VOCs | CAN_1 | CAN_2 |
|---|---|---|
| Carbon dioxide | 0.17 | 0.59 |
| Hexane | − 0.10 | 0.15 |
| Benzaldehyde 2,3,6-thrichloro | 0.00 | 0.52 |
| Acetone | 0.18 | 0.56 |
| Tert butyl alcohol | 0.10 | 0.23 |
| 2-Butanone | 0.50 | 0.24 |
| Isopropyl alcohol | 0.55 | 0.32 |
| Ethanol | 0.04 | 0.18 |
| 2-Pentanone | 0.47 | 0.00 |
| 2-3 Butandione | − 0.16 | 0.26 |
| 2-Propenoic acid,2 methyl-methyl ester | 0.27 | 0.04 |
| Trichloromethane | − 0.06 | 0.61 |
| 4-Heptanone | 0.09 | − 0.18 |
| 1-Methoxy-2-propyl acetate | − 0.14 | 0.18 |
| 3-Penten-1-ol | 0.95 | 0.04 |
| Methylglyoxal | 0.20 | 0.11 |
| Allyl isothiocyanate | 0.21 | 0.06 |
| Nonanal | − 0.21 | − 0.03 |
| Benzene allyl | 0.01 | 0.25 |
| Acetic acid | 0.18 | − 0.04 |
| 1 Hexanol-2-ethyl | − 0.19 | 0.22 |
| Pyrrole | 0.60 | − 0.10 |
| Benzaldehyde | 0.00 | 0.57 |
| Butanoic acid | − 0.09 | − 0.51 |
| Benzaldehyde-4-methyl | 0.01 | 0.11 |
| Valeric acid | − 0.02 | − 0.40 |
| Phenol, 2-4-di-tert-butyl- | − 0.25 | 0.08 |
| Phenol,o-methoxy | 0.04 | 0.19 |
| Dimethylsulfone | − 0.14 | 0.22 |
| Phenol | − 0.03 | 0.21 |
| 2-Ethenylfuran | − 0.12 | 0.16 |
| Phenol,4-methyl- | − 0.20 | − 0.35 |
| Phenol,2–5, bis (1,1-dimethyl-ethyl) | 0.13 | 0.07 |
| Variance explained (%) | 83.04 | 13.10 |
| Cumulative variance (%) | 83.04 | 96.14 |
VOCs with a higher correlation coefficient are shown in italics
Fig. 5.
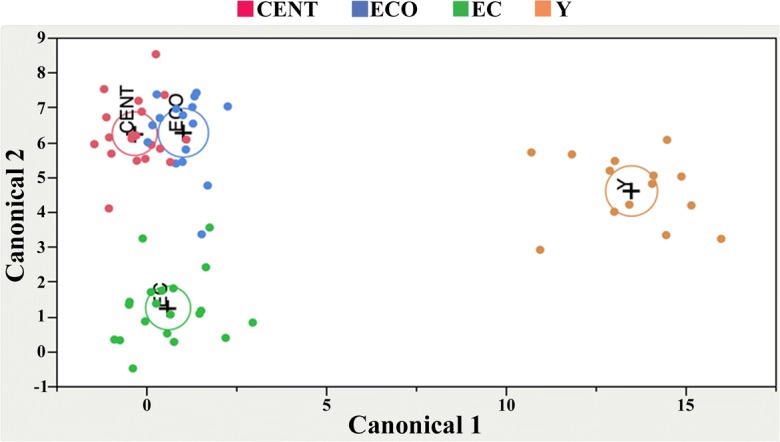
Two-dimensional scatter plot by using canonical discriminant analysis showing the differentiation in VOCs profile from urine of the four age groups: centenarians (CENT), centenarian’s offspring (ECO), elderly controls (EC), young subjects (Y). Canonical_1 variable separated the Y subjects from the other groups. Canonical_2 variable separated CENT and ECO from EC
VOCs signature of familiarity
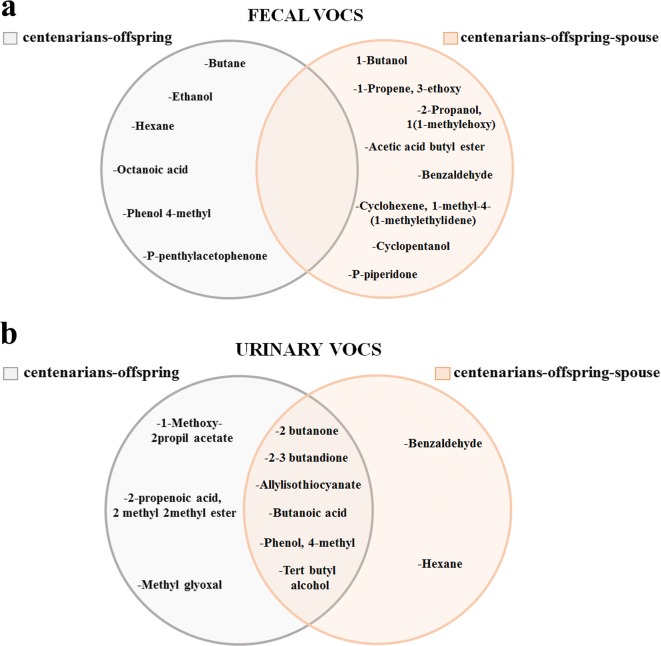
Taking advantage of the presence in our cohort of 10 couples of centenarians and their offspring and 5 family trios (centenarian, his/her offspring, and the spouse of the offspring), we could identify the VOCs that are shared by the couples (likely influenced by genetics) or by the trios (likely influenced by environmental factors, such as shared living environment and diet). The results of the discriminant analysis are reported in Fig. 6. Regarding feces (Fig. 6a), the VOCs that explain the majority of similarity between the couple centenarian–offspring were octanoic acid, ethanol, and phenol 4-methyl, while those explaining the majority of similarity of the family trios (centenarian, offspring and spouse) were cyclopentanol, benzaldeide, and acetic acid butyl ester. As for urine samples (Fig. 6b), the VOCs that explain the majority of similarity of the couple centenarian–offspring were 2-3 butandione, 2-propenoic acid, 2 methyl-methyl ester, and 1-methoxy-2-propyl acetate, while those explaining the majority of similarity of the family trios were 2-butanone and allyl isothiocyanate, tert butyl alcohol, and butanoic acid. As it can be seen in Fig. 6, no overlapping between couples and trios was present for fecal VOCs, while for urinary VOCs, the majority of them was present in both groups, suggesting that these VOCs are likely very common components of urine.
Fig. 6.
Venn diagrams providing a graphical representation of VOCs from feces and urine detectable in the couples (centenarian and his/her offspring) or in the trios (centenarian, his/her offspring, and the spouse of the offspring). a Venn diagram illustrating the VOCs differentially expressed in feces from couples and trios. b Venn diagram illustrating the VOCs differentially expressed in urine from couples and trios
Discussion
While there are several studies on VOCs profiles related to different pathologies, relatively little research has focused on the VOCs profile of aging and longevity. To our knowledge, only the study of Mazzatenta et al. (2015) identified age-specific patterns of VOCs in exhaled breath, focusing in particular on centenarians. However, the normal composition of VOCs in breath may be altered, qualitatively or quantitatively, by stress, time of day, and activity, in addition to others exogenous factors from external environment (Pleil et al. 2013; Filipiak et al. 2016). Therefore, we decided to study the profile of VOCs in other body matrices, such as feces and urine that better mirror the endogenous metabolism of the organism. In particular, in urine, it is possible to find the same metabolites of the plasma, including VOCs, but at a much higher concentration. Therefore, urine represents a useful sample when looking for endogenous VOCs. We then analyzed the profile of VOCs of these two matrices in subjects of different age, from young to centenarians, including elderly that are centenarians’ offspring, and their age-matched controls, these latter being representative of the general elderly population. We have demonstrated for the first time that the profile of VOCs changes with aging and each age group exhibits a different pattern of VOCs, both in feces and urine. These different patterns of fecal and urinary VOCs may likely reflect changes in metabolic processes associated with age and health or inflammatory status.
Among the age-associated changes in VOCs profiles, in our samples, we have found that the fecal VOCs belonging to aldehydes class are less abundant in the Y group compared with the three groups of elders. Metabolites belonging to aldehydes class are detected in diverse body fluids and breath gases, and they are the product of various processes, such as lipid peroxidation, as well as amino acid and carbohydrate catabolism (Das et al. 2016; Dator et al. 2019). Previous studies have shown that aldehydes are produced during inflammatory processes and oxidative stress (Ahmed et al. 2016) and are involved in various diseases, including atherosclerosis, cardiovascular diseases, cancer, and neurodegenerative diseases, as well as metabolic disorders (Kawai et al. 2007; Fuchs et al. 2010; Ahmed et al. 2013; Mochalski et al. 2015; Filipiak et al. 2016). Therefore, the presence of higher level of aldehydes in the groups of elders compared with the young group could be considered an indicator of inflammaging, a state of chronic, low-grade inflammation typical of the elderly (Franceschi et al. 2000, 2007, 2017; Franceschi and Campisi 2014), and used as a new marker for it.
Unlike aldehydes, the fecal compounds belonging to the thiols class resulted more abundant in the Y group with respect to the groups of elders. VOCs belonging to the thiol class contain a sulphydryl group and are present in breath and various biological body matrices, such as blood, urine, and feces (Tangerman 2009; Mochalski et al. 2013). The most representative in feces, as also confirmed by our results, is methanethiol, dimethylsulfide, and dimethyl trisulfide that derive from the methylation of hydrogen sulfide as a detoxification mechanism of gut mucosa (Levitt et al. 1999). Colonic bacteria produce elevated quantities of hydrogen sulfide causing high levels of toxicity and severe damage to gut tissue if the mucosa cannot rapidly metabolize these compounds (Furne et al. 2001). Previous studies have demonstrated that these thiol compounds, derived from the metabolism of hydrogen sulfide, are found in healthy subjects but not in patients with inflammatory bowel diseases (Ahmed et al. 2013, 2016). The higher concentration of thiols in young subjects with respect to the groups of elders may be an indicator of a higher capacity of detoxification of young with respect to old people that are more predisposed to develop various forms of inflammatory bowel diseases.
As far as the classes of anhydrides and esters, they have been found more abundant in feces from ECO group. Ester compounds are volatile fatty acids, with less than six carbon atoms, known also as short-chain fatty acids (SCFAs). SCFAs are produced through the fermentation of not digestible carbohydrates by the gut microbiota and are associated with positive metabolic effects (Polyviou et al. 2016) and modulation of immune-inflammatory reactions through promotion of Treg activity (Ahmed et al. 2016). Therefore, it is not unexpected that esters and SCFAs are more represented in the ECO group, as they may protect from age-associated inflammation, thus accounting at least in part for the better health status of these subjects (Bucci et al. 2016; Chambers et al. 2018; Nagpal et al. 2018).
In urine, only benzenoid class displayed differences among age groups. In particular, we have found higher levels of benzenoid metabolites in the EC group compared with the others. Very limited data are present in literature on the effects of benzenoid metabolites on pathological conditions or aging. Some studies indicate that in urogenital disorders as well as in cancer, there is an accumulation of benzenoids (Adebayo et al. 2018). This suggests that the EC group is characterized by a worst health status with respect to ECO (Chambers et al. 2018; Nagpal et al. 2018). Accordingly, the EC group displayed three times more comorbidities than the ECO group (Table 1).
Discriminant analysis in feces
Discriminant analysis was able to clearly separate the four groups on the basis of the type/quantity of VOCs. This analysis allowed us to identify, both in feces and urine, specific VOCs that discriminate each age group and that may be considered specific biomarkers of aging and longevity.
In feces, formic acid, butyl ester, 4-carene, and 1H-indole, 5 methyl were associated with the CAN_1 variable that markedly separated ECO and CENT from EC. These VOCs were more associated with EC group showing a highly positive correlation with CAN_1. In previous studies, formic acid, butyl ester, 4-carene, and 1H-indole, 5 methyl were found to be higher in subjects with nonalcoholic fatty liver disease (Raman et al. 2013), cancer (Silva et al. 2011, 2017), and gastrointestinal diseases (Garner et al. 2007). Therefore, given their strong association with EC, but much less with ECO and CENT, low concentrations of these VOCs may be considered possible markers of successful aging.
CAN_2 variable markedly separated ECO from CENT. The VOCs that accounted mostly for this discrimination were butanoic acid methyl ester, butanoic acid ethyl ester, butanoic acid 3-methyl 2-methyl propyl ester, and ethanol. Most of these discriminant VOCs belong to esters class that, as discussed above, has anti-inflammatory effects. These VOCs were more associated with the ECO group and therefore could be considered possible markers of age for subjects characterized by a familiar longevity.
CAN_3 variable markedly separated the Y group from the other groups. The VOCs that accounted mostly for this discrimination were dimethyl trisulfide and 1H-indole, 3 methyl. The first one was more associated with Y group, while 1H-indole, 3 methyl was associated with the other three groups. Dimethyl trisulfide, belonging to thiol class, is normally present in feces in healthy conditions, as described above. The ratio between these two VOCs could be therefore considered possible markers of age. As a whole, all these results are summarized at a glance in Table 4 (upper part).
Table 4.
Summary table of the most discriminative volatile organic compounds (VOCs) associated with the different aging phenotypes
| VOC | Association with |
|---|---|
| Feces | |
| Formic acid, butyl ester | Healthy aging and longevity |
| 4-Carene | Healthy aging and longevity |
| 1H-indole, 5 methyl | Healthy aging and longevity |
| Butanoic acid methyl ester | Age (within familiar longevity) |
| Ethanol | Age (within familiar longevity) |
| Butanoic acid ethyl ester | Age (within familiar longevity) |
| Butanoic acid, 3-methyl-, 2-methyl propyl ester | Age (within familiar longevity) |
| Dimethyl trisulfide | Aging |
| 1H-indole, 3 methyl | Aging |
| Urine | |
| 2-Butanone | Aging |
| Isopropyl alcohol | Aging |
| 2-Pentanone | Aging |
| 3-Penten-1-ol | Aging |
| Pyrrole | Aging |
| Carbon dioxide | Healthy aging and longevity |
| Benzaldehyde 2,3,6-thrichloro | Healthy aging and longevity |
| Acetone | Healthy aging and longevity |
| Trichloromethane | Healthy aging and longevity |
| Benzaldehyde | Healthy aging and longevity |
| Butanoic acid | Healthy aging and longevity |
Discriminant analysis of urines
Also in urines we have found discriminant VOCs that differentiate the four age groups. Several studies demonstrated that VOCs from urine are present in patients with different pathologies (Sethi et al. 2013; Gao and Lee 2019). The urinary VOCs that discriminate the four groups of subjects belong in most cases to ketones, aldehydes, alkanes, alcohols, and most of them are associated to lipid metabolism and oxidative stress (Smith et al. 2008; Hakim et al. 2012).
CAN_1 variable markedly separated Y from the other three groups and the discriminant VOCs involved were 2-butanone, isopropyl alcohol, 2-pentanone, 3-penten-1-ol, and pyrrole. These VOCs were detected in the urine of healthy subjects but also, in some cases with higher levels, in patients with different pathologies, such as cancer, nephropathy, and metabolic disorders (Silva et al. 2012; Wang et al. 2017; Mochalski et al. 2018; Jia et al. 2019). In our samples, these VOCs were more associated with Y group as they showed a higher correlation with CAN1. Therefore, they could be considered possible markers characterizing young age.
CAN_2 variable markedly separated EC from ECO and CENT. The VOCs that accounted mostly for this discrimination were carbon dioxide, benzaldehyde 2,3,6-thrichloro, acetone, trichloromethane, benzaldehyde, and butanoic acid. These VOCs, with the exception of butanoic acid, were more associated with ECO and CENT groups, as they showed a higher correlation with CAN_2. Therefore, these VOCs could be considered as possible markers of healthy aging and longevity. As a whole, all these results are summarized at a glance in Table 4 (lower part).
Discriminant analysis of VOCs according to families
Taking advantage of the fact that part of the studied subjects were tied into families (parent–offspring–spouse), we investigated whether some specific VOCs could be considered a familiar trait, i.e., shared by the couple (centenarian–offspring) but not by the trios (centenarian–offspring–spouse), and whether there was an overlapping between couples and trios. This comparison in fact should be able in principle to discriminate between VOCs as inherited trait and VOCs affected by the environment.
As far as fecal VOCs, only six of them resulted present in the analysis of the whole sample after which canonicals were obtained (Table 2 and Fig. 6a). This difference is accounted for by a characteristic of CDA, which selects the minimum number of original variables that maximize the discrimination between groups. Obviously, this selection was related to the composition of the groups: changing the relationship between the members of each groups leads to changes in the number and type of original variables (De Maesschalck et al. 2000). However, it is to note that no overlapping was present between VOCs shared by couples and those shared by trios. This suggests that the familiar component (6 VOCs) is clearly distinguishable from the environmental one (8 VOCs).
As far as urinary VOCs, all those identified as determinant to explain the similarity of the couples and the trios (Fig. 6b) were also present in the analysis of the whole sample after which canonicals were obtained (Table 3), and most of them were shared between the two groups (VOCs of the couples and VOCs of the trios). This suggests that urinary VOCs are likely more conserved among subjects and it is therefore very difficult to find out which ones are really discriminatory for age and/or longevity. In our hands, the only three VOCs that were shared by couples but not trios (and can be therefore suggested as urinary markers of familiar longevity) are methylglyoxal, 1-methoxy-2-propyl acetate, and 2-propenoic acid,2 methyl-methyl ester, the two latter explaining the majority of similarity.
Conclusions
In this study, we have demonstrated for the first time the existence of age-related differences in the pattern of fecal and urinary VOCs, contributing to shed light to complex biology of human aging and longevity. We have observed that the discriminant analysis is able to cluster the subjects based on their age differences, and therefore, VOCs could be considered as promising markers to evaluate aging and longevity. Interestingly, discriminant analysis was also able to discriminate subjects of the same age (EC and ECO), whose only difference was to be offspring of centenarians or not. As ECO are considered to be in a better health state than their age-matched controls, these VOCs could be indicative of a better adaptive capacity, health status, and possible longer life expectancy. Furthermore, some VOCs (especially from feces) are shared between centenarians and their offspring, but not between trios, indicating the possible existence of a familiar component also for VOCs profile.
The main strength of this study is the analysis of two distinct body matrices (feces and urine) in subjects of different age from young to centenarians including centenarians’ offspring and their spouses, a model that allowed us to identify VOCs possibly associated with different phenomena (aging, successful aging and longevity, familiarity, and effect of the environment). We cannot exclude that some differences in VOCs profile are due to environmental conditions, such as diet and socio-economical status. However, as observed with the discriminant analysis of families (that largely shared the living environment), a number of differences emerged when considering couples or trios, indicating that specific VOCs are not affected by a shared environment. At variance, we were not able to evaluate the influence of other individual habits, such as smoking, alcohol consumption, and physical activity, due to the relatively low number of people involved in the study. Another main weakness is the lack of validation of the capacity of these “VOCs signatures” to assign correctly subjects to different classes of age and health status. Ideally, it would be also interesting to test their capability of correlating with individual calendar age and predicting life expectancy. Further studies are needed in this regard. However, at present, these data represent a first indication of the existence of a class of possible biomarkers of age that add up to epigenetic and glycomic clocks, and as a whole, they could open a new avenue of studies on the biology of aging and longevity. In fact, the biological processes that allow centenarians to reach such an advanced age are not yet totally clear. Therefore, the study of VOCs could suggest new and possibly unexplored (or less considered) fields of investigation to uncover/clarify these biological processes.
Electronic supplementary material
(DOCX 46 kb)
Funding information
The study was partially supported by the Roberto and Cornelia Pallotti Legacy for Cancer Research to S.S. and by the Russian Federation mega grant “DPM-AGEING” (grant 074-02-2018-330) on Digitalized and Personalized Medicine of Healthy Aging, 2018–2021, at the Lobachevsky State University of Nizhny Novgorod to C.F.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adebayo AS, Mundhe SD, Awobode HO, Onile OS, Agunloye AM, Isokpehi RD, Shouche YS, Santhakumari B, Anumudu CI. Metabolite profiling for biomarkers in Schistosoma haematobium infection and associated bladder pathologies. PLoS Negl Trop Dis. 2018;12(4):e0006452. doi: 10.1371/journal.pntd.0006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I, Greenwood R, Costello Bde L, Ratcliffe NM, Probert CS. An investigation of fecal volatile organic metabolites in irritable bowel syndrome. PLoS One. 2013;8(3):e58204. doi: 10.1371/journal.pone.0058204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I, Greenwood R, Costello B, Ratcliffe N, Probert CS. Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Aliment Pharmacol Ther. 2016;43(5):596–611. doi: 10.1111/apt.13522. [DOI] [PubMed] [Google Scholar]
- Amann A, Costello Bde L, Miekisch W, Schubert J, Buszewski B, Pleil J, Ratcliffe N, Risby T. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014;8(3):034001. doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- Bacalini MG, Franceschi C, Gentilini D, Ravaioli F, Zhou X, Remondini D, Pirazzini C, Giuliani C, Marasco E, Gensous N, Di Blasio AM, Ellis E, Gramignoli R, Castellani G, Capri M, Strom S, Nardini C, Cescon M, Grazi GL, Garagnani P. Molecular aging of human liver: an epigenetic/transcriptomic signature. J Gerontol A Biol Sci Med Sci. 2019;74(1):1–8. doi: 10.1093/gerona/gly048. [DOI] [PubMed] [Google Scholar]
- Borelli V, Vanhooren V, Lonardi E, Reiding KR, Capri M, Libert C, Garagnani P, Salvioli S, Franceschi C, Wuhrer M. Plasma N-glycome signature of down syndrome. J Proteome Res. 2015;14(10):4232–4245. doi: 10.1021/acs.jproteome.5b00356. [DOI] [PubMed] [Google Scholar]
- Broza YY, Zuri L, Haick H. Combined volatolomics for monitoring of human body chemistry. Sci Rep. 2014;4:4611. doi: 10.1038/srep04611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci L, Ostan R, Cevenini E, Pini E, Scurti M, Vitale G, Mari D, Caruso C, Sansoni P, Fanelli F, Pasquali R, Gueresi P, Franceschi C, Monti D. Centenarians’ offspring as a model of healthy aging: a reappraisal of the data on Italian subjects and a comprehensive overview. Aging (Albany NY) 2016;8(3):510–519. doi: 10.18632/aging.100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collino S, Montoliu I, Martin FP, Scherer M, Mari D, Salvioli S, Bucci L, Ostan R, Monti D, Biagi E, Brigidi P, Franceschi C, Rezzi S. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8(3):e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Pal S, Mitra M. Significance of exhaled breath test in clinical diagnosis: a special focus on the detection of diabetes mellitus. J Med Biol Eng. 2016;36(5):605–624. doi: 10.1007/s40846-016-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dator RP, Solivio MJ, Villalta PW, Balbo S. Bioanalytical and mass spectrometric methods for aldehyde profiling in biological fluids. Toxics. 2019;4:7(2). doi: 10.3390/toxics7020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck R, Jouan-Rimbaud D, Massart DL. The Mahalanobis distance. Chemom Intell Lab Syst. 2000;50(1):1–18. doi: 10.1016/S0169-7439(99)00047-7. [DOI] [Google Scholar]
- Dixon E, Clubb C, Pittman S, Ammann L, Rasheed Z, Kazmi N, Keshavarzian A, Gillevet P, Rangwala H, Couch RD. Solid-phase microextraction and the human fecal VOC metabolome. PLoS One. 2011;6(4):e18471. doi: 10.1371/journal.pone.0018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipiak W, Mochalski P, Filipiak A, Ager C, Cumeras R, Davis CE, Agapiou A, Unterkofler K, Troppmair J. A compendium of volatile organic compounds (VOCs) released by human cell lines. Curr Med Chem. 2016;23(20):2112–2131. doi: 10.2174/092986732366616031612505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab. 2017;28(3):199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S (2018) The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 5(61). 10.3389/fmed.2018.00061 [DOI] [PMC free article] [PubMed]
- Franceschi C, Garagnani P, Gensous N, Bacalini MG, Conte M, Salvioli S. Accelerated bio-cognitive aging in down syndrome: state of the art and possible deceleration strategies. Aging Cell. 2019;18(3):e12903. doi: 10.1111/acel.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer. 2010;126(11):2663–2670. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol. 2001;62(2):255–259. doi: 10.1016/S0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Gao Q, Lee WY. Urinary metabolites for urological cancer detection: a review on the application of volatile organic compounds for cancers. Am J Clin Exp Urol. 2019;7(4):232–248. [PMC free article] [PubMed] [Google Scholar]
- Garner CE, Smith S, de Lacy CB, White P, Spencer R, Probert CS, Ratcliffe NM. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007;21(8):1675–1688. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- Giuliani C, Pirazzini C, Delledonne M, Xumerle L, Descombes P, Marquis J, Mengozzi G, Monti D, Bellizzi D, Passarino G, Luiselli D, Franceschi C, Garagnani P. Centenarians as extreme phenotypes: an ecological perspective to get insight into the relationship between the genetics of longevity and age-associated diseases. Mech Ageing Dev. 2017;165(Pt B):195–201. doi: 10.1016/j.mad.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, Haick H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev. 2012;112(11):5949–5966. doi: 10.1021/cr300174a. [DOI] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Zhunan, Patra Abhijeet, Kutty Viknish, Venkatesan Thirumalai. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites. 2019;9(3):52. doi: 10.3390/metabo9030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Takeda S, Terao J. Lipidomic analysis for lipid peroxidation-derived aldehydes using gas chromatography-mass spectrometry. Chem Res Toxicol. 2007;20(1):99–107. doi: 10.1021/tx060199e. [DOI] [PubMed] [Google Scholar]
- Lee J, Ngo J, Blake D, Meinardi S, Pontello AM, Newcomb R, Galassetti PR. Improved predictive models for plasma glucose estimation from multi-linear regression analysis of exhaled volatile organic compounds. J Appl Physiol. 2009;107(1):155–160. doi: 10.1152/japplphysiol.91657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999;104(8):1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhao N, Wang M, Pi X, Feng Y, Wang Y, Tong H, Zhu L, Wang C, Li E. Urine volatile organic compounds as biomarkers for minimal change type nephrotic syndrome. Biochem Biophys Res Commun. 2018;496(1):58–63. doi: 10.1016/j.bbrc.2017.12.164. [DOI] [PubMed] [Google Scholar]
- Mardia KV. Assessment of multinormality and robustness of Hotelling’s T2 test. Appl Stat. 1975;24:163–171. doi: 10.2307/2346563. [DOI] [Google Scholar]
- Mardia KV, Bookstein FL, Moreton IJ. Statistical assessment of bilateral symmetry of shapes. Biometrika. 2000;87:285–300. doi: 10.1093/biomet/87.2.285. [DOI] [Google Scholar]
- Mazzatenta A, Pokorski M, Di Giulio C. Real time analysis of volatile organic compounds (VOCs) in centenarians. Respir Physiol Neurobiol. 2015;209:47–51. doi: 10.1016/j.resp.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Mochalski P, King J, Klieber M, Unterkofler K, Hinterhuber H, Baumann M, Amann A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst. 2013;138(7):2134–2145. doi: 10.1039/C3AN36756H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochalski P, Theurl M, Sponring A, Unterkofler K, Kirchmair R, Amann A. Analysis of volatile organic compounds liberated and metabolised by human umbilical vein endothelial cells (HUVEC) in vitro. Cell Biochem Biophys. 2015;71(1):323–329. doi: 10.1007/s12013-014-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochalski P, Leja M, Gasenko E, Skapars R, Santare D, Sivins A, Aronsson DE, Ager C, Jaeschke C, Shani G, Mitrovics J, Mayhew CA, Haick H. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J Breath Res. 2018;12(4):046005. doi: 10.1088/1752-7163/aacbfb. [DOI] [PubMed] [Google Scholar]
- Montoliu I, Scherer M, Beguelin F, DaSilva L, Mari D, Salvioli S, Martin FP, Capri M, Bucci L, Ostan R, Garagnani P, Monti D, Biagi E, Brigidi P, Kussmann M, Rezzi S, Franceschi C, Collino S. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging (Albany NY) 2014;6(1):9–25. doi: 10.18632/aging.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. Gut microbiome and aging: physiological and mechanistic insights. Nutr Health Aging. 2018;4(4):267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Stiegel MA, Risby TH. Clinical breath analysis: discriminating between human endogenous compounds and exogenous (environmental) chemical confounders. J Breath Res. 2013;7(1):017107. doi: 10.1088/1752-7155/7/1/017107. [DOI] [PubMed] [Google Scholar]
- Polyviou T, MacDougall K, Chambers ES, Viardot A, Psichas A, Jawaid S, Harris HC, Edwards CA, Simpson L, Murphy KG, Zac-Varghese SE, Blundell JE, Dhillo WS, Bloom SR, Frost GS, Preston T, Tedford MC, Morrison DJ. Randomised clinical study: inulin short-chain fatty acid esters for targeted delivery of short-chain fatty acids to the human colon. Aliment Pharmacol Ther. 2016;44(7):662–672. doi: 10.1111/apt.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povolo M, Contarini G, Mele M, Secchiari P. Study on the influence of pasture on volatile fraction of ewes’ dairy products by solid-phase microextraction and gas chromatography-mass spectrometry. J Dairy Sci. 2007;90(2):556–569. doi: 10.3168/jds.S0022-0302(07)71539-4. [DOI] [PubMed] [Google Scholar]
- Probert CS, Ahmed I, Khalid T, Johnson E, Smith S, Ratcliffe N. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J Gastrointest Liver Dis. 2009;18(3):337–343. [PubMed] [Google Scholar]
- Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, Rioux KP. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(7):868–75.e1-3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc., Cary, NC, United States
- Serra A, Buccioni A, Rodriguez-Estrada MT, Conte G, Cappucci A, Mele M. Fatty acid composition, oxidation status and volatile organic compounds in “Colonnata” lard from Large White or Cinta Senese pigs as affected by curing time. Meat Sci. 2014;97(4):504–512. doi: 10.1016/j.meatsci.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Sethi S, Nanda R, Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. 2013;26(3):462–475. doi: 10.1128/CMR.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150(3):257–266. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- Silva CL, Passos M, Câmara JS. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer. 2011;105(12):1894–1904. doi: 10.1038/bjc.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CL, Passos M, Câmara JS. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers--a powerful strategy for breast cancer diagnosis. Talanta. 2012;89:360–368. doi: 10.1016/j.talanta.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Silva CL, Perestrelo R, Silva P, Tomás H, Câmara JS. Volatile metabolomics signature of human breast cancer cell lines. Sci Rep. 2017;7:43969. doi: 10.1038/srep43969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Burden H, Persad R, Whittington K, de Lacy CB, Ratcliffe NM, Probert CS. A comparative study of the analysis of human urine headspace using gas chromatography-mass spectrometry. J Breath Res. 2008;2(3):037022. doi: 10.1088/1752-7155/2/3/037022. [DOI] [PubMed] [Google Scholar]
- Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877(28):3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Vanhooren Valerie, Dewaele Sylviane, Libert Claude, Engelborghs Sebastiaan, De Deyn Peter Paul, Toussaint Olivier, Debacq-Chainiaux Florence, Poulain Michel, Glupczynski Youri, Franceschi Claudio, Jaspers Koos, van der Pluijm Ingrid, Hoeijmakers Jan, Chen Cuiying Chitty. Serum N-glycan profile shift during human ageing. Experimental Gerontology. 2010;45(10):738–743. doi: 10.1016/j.exger.2010.08.009. [DOI] [PubMed] [Google Scholar]
- von Grafenstein S, Fuchs JE, Huber MM, Bassi A, Lacetera A, Ruzsanyi V, Troppmair J, Amann A, Liedl KR. Precursors for cytochrome P450 profiling breath tests from an in silico screening approach. J Breath Res. 2014;8(4):046001. doi: 10.1088/1752-7155/8/4/046001. [DOI] [PubMed] [Google Scholar]
- Wang M, Xie R, Jia X, Liu R. Urinary volatile organic compounds as potential biomarkers in idiopathic membranous nephropathy. Med Princ Pract. 2017;26(4):375–380. doi: 10.1159/000478782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 46 kb)