Abstract
External length is one of the most conspicuous aspects of mammalian tail morphological diversity. Factors that influence the evolution of tail length diversity have been proposed for particular taxa, including habitat, diet, locomotion and climate. However, no study to date has investigated such factors at a large phylogenetic scale to elucidate what drives tail length evolution in and across mammalian lineages. We use phylogenetic comparative methods to test a priori hypotheses regarding proposed factors influencing tail length, explore possible interactions between factors using evolutionary best-fit models, and map evolutionary patterns of tail length for specific mammalian lineages. Across mammals, substrate use is a significant factor influencing tail length, with arboreal species maintaining selection for longer tails. Non-arboreal species instead exhibit a wider range of tail lengths, secondarily influenced by differences in locomotion, diet and climate. Tail loss events are revealed to occur in the context of both long and short tails and influential factors are clade dependent. Some mammalian groups (e.g. Macaca; primates) exhibit elevated rates of tail length evolution, indicating that morphological evolution may be accelerated in groups characterized by diverse substrate use, locomotor modes and climate.
Keywords: tail loss, tail length, phylogenetic comparative methods, vertebral column, allometry
1. Introduction
Tails are a conspicuously diverse appendage among mammals, and tail length is one of the most noticeable and well-studied aspects of its morphological variation [1–8]. The presence of a tail is the vertebrate ancestral condition [1,9], and variation in tail length relative to head and body length (as well as tail presence versus absence) varies widely among mammals and is both functionally and phylogenetically informative [10–12]. Individual taxa (e.g. cavies [Rodentia], wombats [Diprotodontia]) as well as some entire clades (e.g. hominoids [apes and humans] [Primates]) lack externally visible tails, while other taxa may have tails that are as long or longer than the combined length of the head and body (e.g. jerboas [Rodentia], sugar gliders [Diprotodontia], galagos [Primates]) [13,14].
Various ecological and behavioural factors have been proposed to influence mammalian tail phenotypic diversity, including substrate use (e.g. inherent need for balance and stability in arboreal settings) [15–20], locomotion (e.g. for dynamic stability, propulsion, manoeuvrability, prehensility) [21–27], diet (e.g. in predatory behaviours) [28–30] and climate (e.g. for thermoregulation) [31–35]. Because long tails are observed in arboreal or semi-arboreal quadrupedal mammals, such as rodents [4,16,17,19], primates [3,18,20,36,37] and tree shrews [38], whereas their close phyletic terrestrial relatives have shorter tails, tail length is presumably related to the tail's use (or not) as a static counterweight or in creating angular momentum for maintaining balance during arboreal postures and locomotion [16–20,36–38]. In this scenario, long tails are maintained because arboreal substrates are inherently more dangerous than the ground as supports are smaller/narrower relative to body size, discontinuous and less stable (i.e. compliant); therefore, falling from the height of a tree may lead to injury and/or death, impacting an animal's fitness [39]. Mammals with specialized forms of arboreal locomotion may also require long tails: a longer tail in gliding species may aid in manoeuvrability during takeoff [23], and a long tail with well-developed distal muscle mass and prehensile ability can support the weight of a suspended body [40].
Mammals that rely on other forms of locomotion or substrates (i.e. aerial, aquatic, semi-aquatic, subterranean, terrestrial) presumably do not experience these same selective pressures for balancing mechanisms during positional behaviours, and the tail may be ‘freed' to serve alternate functions, potentially driving evolutionary changes in tail morphology including length. Long tails are used as a propellers or rudders during swimming in otters and beavers [41,42], provide propulsive force during bipedal hopping in kangaroos and wallabies [21,22,27], and can be used as rudders for fast changes in direction during predatory behaviours in carnivorous species such as bats or cheetahs [15,24,29,43]. However, long tails could be disadvantageous for herbivorous species such as rabbits, terrestrial squirrels and mice [4,44] as it may be easily grasped by predators during evasive behaviours. Subterranean species (e.g. tuco-tuco, moles) do not have a need for speed, and in fact long tails could hinder movements during burrowing [45,46]. Furthermore, tail length has been hypothesized to differ depending on climate, as it serves as a site for heat dissipation or conservation by increasing (longer tails) or restricting (shorter tails) surface area, respectively [3,32,34,47]. Behaviourally, tails have been observed to serve as ‘blankets' to trap heat [33] or as a ‘parasol' to provide shade [31].
The impacts of various ecological and behavioural factors on tail length diversity have been demonstrated by researchers studying individual taxa or clades (e.g. Carnivora [48], Primates [2,3,5,18,20,36,49,50], Rodentia [4,16,17,19,33] and Scandentia [38]). However, the shared selective pressures and constraints governing observed differences in tail length have not been explored in a broader phylogenetic context to elucidate how this observed phenotypic diversity evolved among and within mammalian clades. The goal of this study is to systematically evaluate the macroevolution of mammalian tail length diversity at a large phylogenetic scale using formal phylogenetic comparative methods to (1) test a priori hypotheses about the ecological and behavioural variables that influence tail morphology, both within and across all mammalian orders, (2) create models of best fit for tail length evolution to explore possible interactions between these proposed variables and (3) document patterns of tail length diversity along specific mammalian lineages.
2. Material and methods
(a). Mammalian sample and phylogeny
The study sample consists of 1244 mammalian species with available morphometric data (see below). All analyses were performed using the most recently available mammalian phylogeny constructed by Smaers et al. [51]. The data are considered phylogenetically representative as the number of species collected for each order matches the per cent of species it represents out of the total known mammalian species.
(b). Tail morphological data
Quantitative and qualitative data on tail morphology were taken from the literature [13,14]. We obtained measures of tail length (mm) (herein ‘TL'), and two body size variables including head + trunk length (mm) (i.e. body length herein ‘BL') and body mass (gm) (herein ‘BM'). All analyses were performed on sex-pooled average measurements for each species. Although analysing each species use sex-specific measurements might improve the resolution of this study, these measurements were not distinguished in the literature used for data collection [13,14].
Previous studies have looked at differences in tail length using relative tail length (RTL = tail length/head + trunk length × 100) [2,5,7]. Here, RTL was employed in our examination of the effects of climate on TL because it allowed us to account for body size when looking at the relationship between TL and latitude. While RTL is an established appropriate measure for quantifying tail length [2,5,7], it does not allow us to explicitly look at how TL scales to BL or BM [52]. Previous work suggests that TL scales negatively with body size for particular mammalian groups [49,53,54] and this relationship has not been examined at a larger phylogenetic scale. To determine how TL may scale allometrically with metrics of body size across phylogeny, we used pANCOVA to analyse TL against body size measures (rather than RTL), which allows us to directly test if functional groups deviate from allometric predictions [4,49,52–55].
(c). Behavioural and ecological variables of investigation
We generated a list of behavioural and ecological variables and categorical states hypothesized to influence mammalian tail length evolution based on the literature (electronic supplementary material). Categorical factors include substrate use, locomotor type, diet and tail prehensility.
(d). Data analysis
One limitation of using phylogenetic comparative methods that is of considerable importance to the present study is that they assume normally distributed data. To satisfy this assumption, all species without an externally visible tail (i.e. tailless) had to be removed from the initial analysis because they are considered ‘outliers’ and would violate this assumption if included. Therefore, all described statistical analyses and modelling procedures were conducted without tailless species. However, because tailless taxa are of obvious importance to this investigation of tail length reduction, they were subsequently included on the phylogenetic tree to allow us to visualize the phylogenetic context of tail loss events.
(i). A priori hypothesis testing: phylogenetic generalized least squares
We first examined the relationships between TL, BL and BM using phylogenetic generalized least-squares (pGLS) regressions [56,57] (electronic supplementary material). This method allowed us to test for allometric differences in tail length and see how larger bodies (either mass or length) might affect tail length depending on the categorical groups tested. pGLS is a modified generalized least-squares regression that incorporates phylogenetic data to estimate and account for covariation expected by the relatedness between species [55,58] (see electronic supplementary material for more details).
(ii). A priori hypothesis testing: phylogenetic ANCOVA
We then examined a priori hypotheses by testing for differences in allometry between groups defined by behavioural and ecological variables (electronic supplementary material) to determine if these factors are significantly influencing mammalian tail length. We accomplished this by using phylogenetic analysis of covariance (pANCOVA). A pANCOVA differs from a standard least-squares ANCOVA because it includes the phylogenetic variance–covariance in the error term, which is required to account for phylogeny to evaluate if the slopes and/or intercepts are homogeneous among the groups defined by categorical variables [55]. The pANCOVA tests whether a model with multiple slopes and/or intercepts (i.e. a multi-grade model) provides a significantly better fit than a model using only a single slope and intercept (i.e. a single-grade model). Together, the phylogenetic regression and subsequent pANCOVA can test if species deviate from allometric predictions based on the different behavioural and ecological variables of interest [55] (see electronic supplementary material for more details).
(iii). Evolutionary modelling
We created a best-fit model of evolution to fit adaptive models of tail length evolution. Unlike pANCOVA, the modelling procedure only employs quantitative measures of TL and BL, which allowed us to test for shifts in TL∼BL independent of the categorial variables defined in our a priori hypotheses. We obtained a best-fit evolutionary model using multi-optima Ornstein Uhlenbeck (OU) [59], which identifies shifts in slope and/or intercept in the evolutionary allometry using an MCMC reversible-jump algorithm. Shifts in slope and intercept proposed by this model estimation approach were translated to a least-squares framework and tested for significance. The output of the procedure is a detailed evolutionary model of the bivariate relationship between tail length and body length evolution (see electronic supplementary material for more details). All shifts in slope and intercept highlighted in the evolutionary model were subsequently assessed using pANCOVA to test whether including each additional regime provides a statistically better fit to the data than a model with fewer groups.
The groups designated by the best-fit model can then be compared to the results of our a priori hypothesis testing to see how ecological and behavioural variables may interact differently in select clades and/or species. For example, if the regimes highlighted in the model align with the groups for a particular variable of interest (substrate, locomotion, diet etc.), this finding can provide support for the relatively greater influence of that particular factor on tail length evolution. On the contrary, if the regimes highlighted in the best-fit evolutionary model do not align well with the groups from one of our tested variables, we can instead assess how ecological and behavioural variables may have different levels of influence in different parts of the tree, and how the variables interact to influence tail length evolution.
(iv). Patterns of tail length evolution
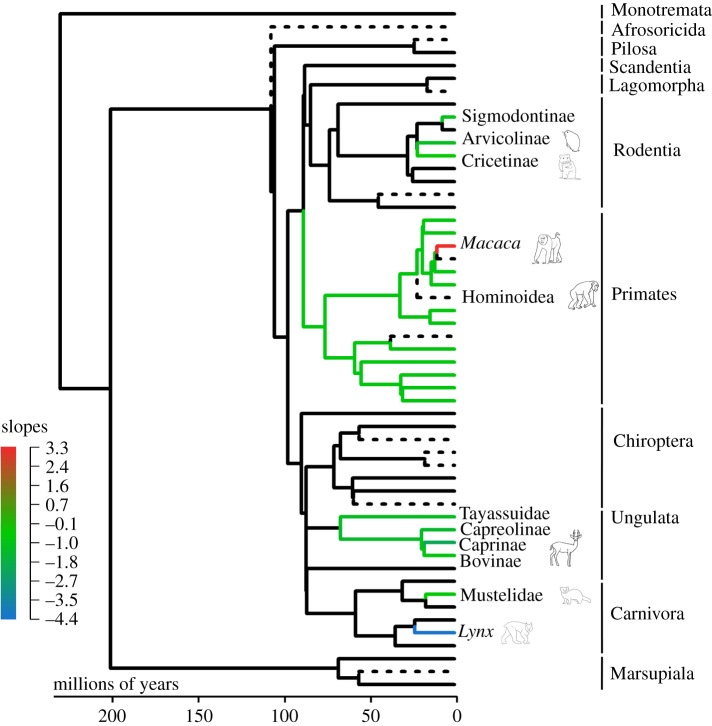
To infer patterns of mammalian tail length macroevolution, we used the results from the best-fit model to visualize where shifts in tail length have occurred in the phylogeny, and how tail length evolution may differ among or within clades. Although not a statistical test, mapping evolutionary patterns of tail length diversity is especially important for understanding the phylogenetic context of tail loss events. As mentioned above, tailless species could not be included in the modelling procedures without violating assumptions of normality, so they were subsequently added onto the resulting best-fit model in order to visualize the tail length condition of species closely related to tailless species (figure 2). Visualizing the phylogenetic context of tail loss events can lend answers to questions about whether there was a gradual decrease in tail length or if it was a sudden loss event, and how tail loss evolution may be influenced by different factors.
Figure 2.
Summary tree of the evolutionary best-fit model for tail length ∼ body length. Black represents the ancestral condition and each colour represents a statistically significant evolutionary shift (regime) in slope and intercept (available in electronic supplementary material), coloured by the slope for each group. Tailless species (dashed lines) were not included in the evolutionary best-fit model but have been subsequently added back onto the tree to show where tail loss has occurred in phylogeny.
Finally, to explore differences in the process of tail length evolution, we completed rate analyses to compare the amount of phenotypic change between the groups proposed by the best-fit model [60]. This method calculates the net rates of trait evolution to look for differences in the pace of phenotypic evolution in particular groups [61–63] (see electronic supplementary material for more details). Quantifying the rates of tail length evolution in different groups may highlight how tail phenotypic diversity is produced and differences may reflect processes such as selection, drift and adaptive radiations that may all be influenced by several behavioural or ecological variables [62,64,65].
3. Results and discussion
(a). Tail length in relation to total body length and body mass
Studies of body size in primates have demonstrated that tail length generally scales against body mass with negative allometry, such that larger-bodied primates have relatively shorter tails [49,53,54]. The results of both a priori hypothesis testing and evolutionary modelling support negative allometry between tail length (TL) and body size (i.e. BL, BM) among all mammals (slopes less than 1 for TL∼BL, less than 0.33 for TL∼BM) (electronic supplementary material, tables S1 and S2). As body mass and body length increase, tail length increases at a slower rate than expected, indicating that tail length is more limited at larger body sizes. A larger body with a proportionally larger or heavier tail may be difficult to manoeuvre and/or energetically maintain [49,53]. Mechanical constraints of large body size (e.g. on leaping) may also lead to adaptive differences, such as increased terrestriality, which may have relaxed selection on the tail for maintaining balance leading to reduced tail lengths [66].
Exceptions to this trend include quadrupedal hoppers (i.e. rabbits and hares), as well as the evolutionary regimes that comprise bovines (Artiodactyla) and Lynx (Carnivora) (electronic supplementary material, tables S1–S3). While species in these groups have relatively shorter tails than other mammals, they exhibit positive allometric relationships between tail length and body size. Tail length in some of these groups may be influenced by factors that were not included in this study. For example, artiodactyls have been documented to use their tails as fly swatters which may select for a longer tail than expected to provide further reach in larger species [1,67].
(b). Ecological and behavioural variables correlated with tail length
Substrate use, locomotor mode, diet and climate all significantly impact mammalian tail length (figure 1; electronic supplementary material, table S1). Results of a priori hypothesis testing were similar when accounting for either BL or BM as our measure of body size for most ecological and behavioural groups. The results for BL are discussed in detail below (see electronic supplementary material for all results). Groups with different results for BL and BM are described within the context of the ecological or behavioural variable.
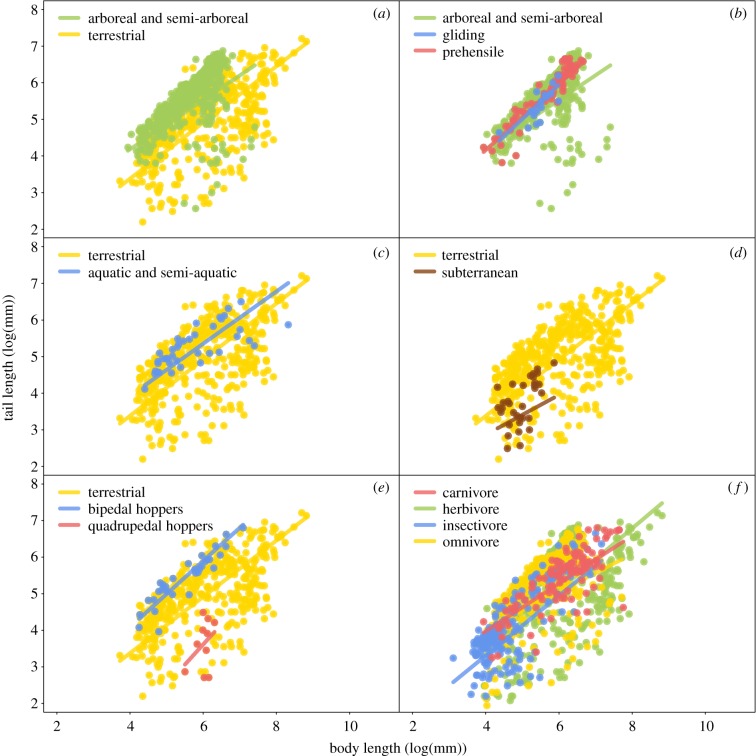
Figure 1.
Tail length ∼ body length for the behavioural and categorical variables tested. Data points and lines of best fit shown for statistically significant differences (p < 0.05) in lines of best fit for substrate (a,c), locomotor (b,d,e) and dietary (f) groups. All results listed in electronic supplementary material, table S1. Arboreal and semi-arboreal mammals have significantly longer tails than other mammals (a), and gliding and prehensile-tailed mammals have the longest tails of all arboreal and semi-arboreal mammals (b). Among non-arboreal mammals, aquatic and semi-aquatic species have some of the longest tails (c), while subterranean have the shortest (d). Grouped by locomotor mode, bipedal hoppers have the longest tails and quadrupedal hoppers have the shortest tails (e). Tail length differs by diet, with separation among herbivores, carnivores, omnivores and insectivores (f).
Consistent with trends of substrate use observed for select taxonomic groups documented in the literature, we found that arboreal and semi-arboreal species have significantly longer tails than non-arboreal species (pANCOVA: p < 0.001) (figure 1a; electronic supplementary material, table S1). Long tails are likely more effective than short tails in functioning as a counterweight to aid in balance and manoeuvrability during arboreal postures or movements [15–20,35]. Thus, natural selection likely maintains selection for longer tails in arboreal species in order to safely navigate and maintain balance on small branches and discontinuous supports [17,18,20,36].
Among arboreal and semi-arboreal mammals, prehensile tails exhibit a significantly higher slope than non-prehensile tails (pANCOVA: p < 0.001; figure 1b; electronic supplementary material, table S1), suggesting that as body size increases in prehensile-tailed species, tail length increases at a faster rate than in other arboreal species. Prehensile-tail suspension is a specialized form of locomotion that has independently evolved in lineages from multiple mammalian clades, such as in rodents, marsupials, primates and carnivorans, and has unique osteological and myological correlates associated with increased musculature and mass needed to support the weight of the body during tail-assisted suspension [13,25,26,40,68,69]. Although a longer, heavier tail may be more difficult to manoeuvre and energetically maintain [49,53], the increased musculature of a long, prehensile tail may be especially useful to large-bodied animals living in environments with more structurally fragile forests, where the tail can aid in distributing body weight across multiple supports during locomotion beneath branches [70]. A tail with prehensile ability can also allow an animal, particularly a large-bodied animal, to grab vegetation or to stop and/or slow a fall [70]. Thus, selection for longer tails may be maintained in large-bodied primates and carnivorans that can use their tail as a ‘fifth limb'.
Gliding species were expected to have longer tails than other arboreal species to aid in manoeuvrability, speed and descent [4]. The tail is used to balance and stabilize the body during launch [23], and gliding squirrels typically have a lower body mass and longer tails to help lower their wing-loading (body mass/wing area) and improve gliding aerodynamics [4]. The results of this study indicate that the tail lengths of gliding species have a statistically higher slope when accounting for body length (pANCOVA: p < 0.05) (figure 1b; electronic supplementary material, table S1). Tail length in gliding mammals increases at a faster rate than in other arboreal species. A shorter tail may be satisfactory for generating the necessary forces for takeoff in small, gliding species, but a longer tail may be more beneficial as body size increases [23].
Unlike arboreal mammals, non-arboreal taxa (i.e. aerial, aquatic, semi-aquatic, subterranean, terrestrial) likely do not experience the same strong selection for long tails, and other behavioural and ecological selective pressures may instead generate changes that lead to tail length diversity [35]. Non-arboreal species exhibit a range of both short and long tails depending on substrate, locomotor and dietary preferences. Aquatic and semi-aquatic species (e.g. otters, beavers, aquatic shrews, water mice) have longer tails than species in other non-arboreal substrate groups (pANCOVA: p < 0.001) (figure 1c; electronic supplementary material, table S1). Although some aquatic mammals have additional adaptations that improve their swimming abilities, the tail often aids in swimming via propulsion and lift through water, and increased tail length may improve its ability to act as a strong rudder [41]. Subterranean species have the shortest tails out of all the non-arboreal mammals examined (pANCOVA: p < 0.001) (figure 1d; electronic supplementary material, table S1) [46,71], consistent with the premise that while the tail does have some tactile use underground, a shorter tail is less likely to interfere when an animal is digging and kicking dirt behind them [45]. In addition, underground tunnels are often narrow and burrowing animals must be adept at ‘backing up' rather than ‘turning around', making a short tail unlikely to drag across the ground or interfere with limbs [46].
Within non-arboreal mammals grouped by locomotor mode, bipedal hoppers (e.g. kangaroos, wallabies) have the longest tails (pANCOVA: p < 0.001), and quadrupedal hoppers (e.g. rabbits, hares) the shortest tails (pANCOVA: p < 0.001) (figure 1e; electronic supplementary material, table S1). Bipedal hoppers use the tail as a counterbalance and propulsive organ during locomotion, and as a prop during resting tripod stance [27]. These behaviours likely require a longer, larger tail that can support and/or provide propulsive force to move the large body of these taxa [21,22,27]. By contrast, a long tail may be a liability during predator escape for smaller-bodied quadrupedal hoppers because it can be more easily be grabbed by predators, and could also increase drag and slow the animal down during escape [4].
The impact of predation was also apparent when differences in tail length dependent on diet were considered. We found statistically significant separation in tail length among carnivorans, insectivores, herbivores and omnivores (p < 0.001; electronic supplementary material, table S1; figure 1f). However, we also found that diet is largely influenced by substrate use, and that there is a large spread of tail length values for each dietary group that results in considerable overlap among these dietary groups.
The relationship between RTL and absolute latitude values (our proxy for climate [3,72]) is negative (albeit weak; R2 = 0.19, slope = −1.32, p < 0.001) (electronic supplementary material, figure S4). Mammals living near the equator exhibit greater tail length diversity compared to mammals living further from the equator, which are instead restricted to shorter tail lengths. Numerous hypotheses have sought to explain the role of the tail in thermoregulation, such as serving as a site for heat loss [34,47,73–75], as a parasol to shade the body from the intense sun [31], or as a blanket to create an extra layer of insulation around the body [33]. While dissipation of heat is favourable in warm environments, conservation of heat is necessary in colder climates, and animals may decrease heat loss from the body by reducing appendages, including the length of the tail [2,3,35,76]. Our results suggest that there is selection for shorter tails in mammals living in colder climates. Furthermore, while the tail may still be used to dissipate heat or shade the body in some species, temperature is not necessarily selecting for longer tails in warmer regions. One major caveat is that these results may be influenced by other factors, such as differences in the distribution and diversification of mammals across the globe. Mammals are more likely to inhabit and diversify in warmer regions near the equator [77], which statistically provides more opportunity for differences in tail length and morphology.
(c). Evolutionary best-fit model
To explore tail length evolution independent of our a priori hypotheses, evolutionary best-fit models allow us to visualize where significant shifts in tail length have occurred across mammalian phylogeny [59]. The evolutionary model may also highlight which factors have a stronger influence on tail length within certain mammalian clades, and the possible interplay between factors. The evolutionary model proposed a multi-grade model of best fit with 11 supported regimes, with each regime signalling a statistically significant shift in relative tail length from the ancestral condition (electronic supplementary material, table S3; figure 2). The model did not separate groups based solely on any one of our proposed factors that are presumed to influence mammalian tail length, and instead indicates that the factors influencing tail length evolution affect groups differently. While some factors affect tail length at the order level (e.g. Primates), others further act at the level of family, subfamily or even genus (e.g. Macaca within Primates).
Almost every regime indicated by the evolutionary model comprises non-arboreal mammals, while most arboreal and semi-arboreal species were grouped together into the ancestral regime (figure 2). This result provides compelling evidence that substrate use drives the macroevolution of mammalian tail length diversity. While arboreal locomotion maintains strong selection for long tails, non-arboreal taxa exhibit diverse tail lengths depending on other behavioural and ecological factors. Among mammals, non-arboreal species were often separated from closely related arboreal species and allocated to their own group. For example, the terrestrial (and partially burrowing) subfamilies Arvicolinae (e.g. lemmings, voles) and Cricetinae (e.g. hamsters) have significantly shorter tails than other rodents and were given their own group designations within the order Rodentia.
The notable exception to this trend is in Primates, in which the entire extant order (apart from Macaca, described below) has overall longer tails than the estimated mammalian ancestral condition. Although larger-bodied catarrhines often spend more time on the ground, primates are largely arboreal or semi-arboreal [78,79]. Placed into their own evolutionary regime (as well as one additional regime that will be further discussed), this result for Primates highlights their unique evolutionary history and locomotor adaptations compared to other mammals. Tail length variation among primates was recently examined using evolutionary modelling [80]; however, this study did not examine the evolution of primate tail length within the context of all mammals and not all regime shifts identified were supported by pANCOVA. Furthermore, the authors of this study [80] employed a different procedure for obtaining a best-fit evolutionary model, which included tailless primates in their analysis and therefore violated the assumption of normally distributed data (see methods for more information). These methodological differences explain why fewer evolutionary shifts in primate tail length were identified in our more comprehensive study.
Although the best-fit evolutionary model from this study identified an evolutionary shift toward longer tails at the base of Primates, it also identified a subsequent shift in macaques. Macaques represent one of the most diverse genus of Old World monkeys, comprising at least 22 species [13]. Tail length varies widely within the genus Macaca (e.g. M. fascicularis: tail longer than head + body; M. sylvanus: very short tail), and this variation has been related to climate [2,3,81] and terrestriality [37]. Macaques are distributed over the largest geographical range of all non-human primates, and are adapted to a variety of environmental conditions [2,3,82,83]. Tail length variation in the genus is generated by differences in both the number and length of caudal vertebrae which are likely influenced by distinct mechanisms among macaque groups and should be further described [81,84].
(d). Patterns of tail length evolution and tail loss
Some mammalian clades, including our own (Hominoidea) are diagnostically characterized by the lack of an external tail. To better understand the phylogenetic context of tail loss, we grafted tailless species onto the phylogenetic tree of the best-fit model to visualize the tail length condition occurring at hypothesized periods of tail loss events (figure 2). Tail loss occurs in almost every mammalian order and can evolve in the context of short or long tails. Like tail length reduction, tail loss appears to be influenced by a variety of ecological and behavioural factors but is most common in non-arboreal species.
Tailless species that are primarily arboreal are found within Primates, Pilosa and Diprotodontia (Marsupiala). Tailless arboreal species are often large-bodied, which might impose difficulties for finding arboreal supports large and sturdy enough to support their body weight, thereby leading to increased reliance on the fore- or hindlimbs, or adapting to underbranch locomotor behaviours such as forelimb suspension (i.e. hominoids) or inverted quadrupedalism (i.e. sloths) [53,66,85]. As these forms of locomotion have probably decreased reliance on balance and stability, selection for a long tail is no longer maintained.
Available anatomical evidence suggests early hominoids lacked tails, were large-bodied, and relied predominantly on arboreal quadrupedalism (e.g. Ekembo and Proconsul: 23–16 Ma) rather than more orthograde positional behaviours observed or inferred for crown clade members [10,86,87]. Thus, a tail would have presumably conferred an advantage for navigating relatively small (compared to body size) arboreal supports in early hominoids [7]. Perhaps not surprisingly then, tail loss evolution in hominoids does not appear to be influenced by the previously proposed ecological and behavioural factors examined here. Future studies should continue to explore tail loss in arboreal species (including from genetic or developmental standpoints) given the tail's role in balance and stability, as well as further detail how arboreal mammals behaviourally or anatomically compensate for the loss of the tail [16–20,36].
Tail length evolution was further examined by analysing evolutionary rates in the regimes identified by the best-fit evolutionary model (electronic supplementary material, table S3). A higher evolutionary rate indicates increased levels of phenotypic diversity within the group, which can help drive evolutionary change. Almost all regimes identified by the model had faster evolutionary rates than the ancestral regime, indicating increased levels of phenotypic diversity. Phenotypic diversity in tail length may reflect decreased selection on tail length, allowing for short tails or tail loss to persist in particular lineages. Contrary to expectations, primates (with the exception of macaques) do not have a significantly faster evolutionary rate than the ancestral condition. However, the genus Macaca has the fastest rate out of all regimes tested (electronic supplementary material, table S3). The evolutionary rate in macaques is also significantly higher than all other primates which underscores their complex evolution of phenotypic diversity in the context of a relatively short evolutionary history [88].
(e). Conclusion and future directions
This study tested a priori hypotheses and generated evolutionary best-fit models to generate a holistic view of the factors influencing mammalian tail length evolution. Substrate use influences tail length across all mammals, and the use of arboreal substrates maintains selection for longer tails. By contrast, use of non-arboreal substrates is linked with diversification of environments and behaviours, which can select for either long or short tails. However, although tail loss is more commonly found in non-arboreal species, some arboreal species are characterized by the loss of the tail. Tail loss in these groups may be partially explained by increased body size, or by locomotor modes such as suspension or slow-climbing that have less reliance on balance and stability. However, these factors probably do not explain tail loss within hominoids. Questions about how and why tail loss occurred in our own lineage remain.
Rates of phenotypic evolution differ considerably within and among mammalian clades, providing further insight into how morphological diversity is generated and maintained. This finding suggests that the factors driving tail length evolution can act within lineages to influence the rate of evolution and amount of observed biological diversity. Future studies might compare evolutionary rates for multiple phenotypic traits to see if certain traits evolve faster than others within clades (e.g. tail length versus limb length), which can allow us to see how particular factors may have greater influence on certain regions of the body compared to others.
One limitation to this project is the paucity of information regarding tail use behaviour in different mammalian species. While previous work has recognized the various functions of the tail, more detailed quantitative studies of tail use or ‘behaviour' during various activities will improve the resolution of data available to test the correspondence between mammalian behaviour and tail morphology, including length. In addition, there may be other specializations in tail function not addressed here which might lend answers to questions regarding tail morphological diversity across mammals. Finally, the evolution of any trait results from interactions between both external (i.e. behaviour and environment) and intrinsic (i.e. genetic and developmental) selective pressures. While previous work has largely focused on the environmental and behavioural factors influencing tail morphology, future work should focus on the genetic and developmental mechanisms underpinning tail length variation to provide new insight into tail morphological diversity among mammals.
Supplementary Material
Acknowledgements
We thank Jeroen B. Smaers for helpful discussions and guidance in designing analyses and creating evolutionary models. We also thank John Fleagle and two anonymous reviewers for providing helpful comments on the manuscript, and Lydia Myers for assisting in collection of latitude data.
Data accessibility
This article has no additional data.
Authors' contributions
S.T.M. and G.A.R. conceived and designed the study. S.T.M. collected the data and designed and performed the analysis. Both authors discussed results and contributed to the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Hickman GC. 1979. The mammalian tail: a review of functions. Mamm. Rev. 9, 143–157. ( 10.1111/j.1365-2907.1979.tb00252.x) [DOI] [Google Scholar]
- 2.Fooden J. 1997. Tail length variation in Macaca fascicularis and M. mulatta. Primates 38, 221–231. ( 10.1007/BF02381611) [DOI] [Google Scholar]
- 3.Fooden J, Albrecht GH. 1999. Tail-length evolution in fascicularis-group macaques (Cercopithecidae: Macaca). Int. J. Primatol. 20, 431–440. ( 10.1023/A:1020556922189) [DOI] [Google Scholar]
- 4.Hayssen V. 2008. Patterns of body and tail length and body mass in Sciuridae. J. Mamm. 89, 852–873. ( 10.1644/07-MAMM-A-217.1) [DOI] [Google Scholar]
- 5.Russo GA. 2015. Postsacral vertebral morphology in relation to tail length among primates and other mammals. Anat. Rec. 298, 354–375. ( 10.1002/ar.23004) [DOI] [PubMed] [Google Scholar]
- 6.Russo GA. 2016. Comparative sacral morphology and the reconstructed tail lengths of five extinct primates: Proconsul heseloni, Epipliopithecus vindobonensis, Archaeolemur edwardsi, Megaladapis grandidieri, and Palaeopropithecus kelyus. J. Hum. Evol. 90, 135–162. ( 10.1016/j.jhevol.2015.10.007) [DOI] [PubMed] [Google Scholar]
- 7.Russo GA, Shapiro LJ. 2011. Morphological correlates of tail length in the catarrhine sacrum. J. Hum. Evol. 61, 223–232. ( 10.1016/j.jhevol.2011.03.006) [DOI] [PubMed] [Google Scholar]
- 8.Thorington RW., Jr 1970. Lability of tail length of the white-footed mouse, Peromyscus leucopus noveboracensis. J. Mamm. 51, 52–59. ( 10.2307/1378531). [DOI] [PubMed] [Google Scholar]
- 9.Handrigan GR. 2003. Concordia discors: duality in the origin of the vertebrate tail. J. Anat. 202, 255–267. ( 10.1046/j.1469-7580.2003.00163.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward CV, Walker A, Teaford M. 1991. Proconsul did not have a tail. J. Hum. Evol. 21, 215–220. ( 10.1016/0047-2484(91)90062-Z) [DOI] [Google Scholar]
- 11.Williams SA, Russo GA. 2015. Evolution of the hominoid vertebral column: the long and the short of it. News Rev. 24, 15–32. ( 10.1002/evan.21437) [DOI] [PubMed] [Google Scholar]
- 12.Nakatsukasa M. 2003. Definitive evidence for tail loss in Nacholapithecus, an East African Miocene hominoid. J. Hum. Evol. 45, 179–186. ( 10.1016/S0047-2484(03)00092-7) [DOI] [PubMed] [Google Scholar]
- 13.Nowak RM. 1999. Walker's mammals of the world, 6th edn Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- 14.Mittermeier RA, Rylands AB, Wilson DE. 2013. Handbook of the mammals of the world. Barcelona, Spain: Lynx Editions. [Google Scholar]
- 15.Adams RA, Snode ER, Shaw JB. 2012. Flapping tail membrane in bats produces potentially important thrust during horizontal takeoffs and very slow flight. PLoS ONE 7, e32074 ( 10.1371/journal.pone.0032074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck CW, Tolman N, Tolman W. 1925. The tail as a balancing organ in mice. J. Mamm. 6, 267–271. ( 10.2307/1373415) [DOI] [Google Scholar]
- 17.Horner BE. 1954. Arboreal adaptions of Peromyscus with special reference to use of the tail. Contrib. Lab. Vert. Biol. 61, 1–84. [Google Scholar]
- 18.Igarashi M, Levy JK. 1981. Locomotor balance performance of short-tailed squirrel monkeys. J. Med. Primatol. 10, 136–140. ( 10.1159/000460064) [DOI] [PubMed] [Google Scholar]
- 19.Siegel MI. 1970. The tail, locomotion and balance in mice. Am. J. Phys. Anthropol. 33, 101–102. ( 10.1002/ajpa.1330330113) [DOI] [Google Scholar]
- 20.Young JW, Russo GA, Fellmann CD, Thatikunta MA, Chadwell BA. 2015. Tail function during arboreal quadrupedalism in squirrel monkeys (Saimiri boliviensis) and tamarins (Saguinus oedipus). J. Exp. Zool. Ecol. Genet. Physiol. 323, 556–566. ( 10.1002/jez.1948) [DOI] [PubMed] [Google Scholar]
- 21.Dawson TJ, Taylor CR. 1973. Energetic cost of locomotion in kangaroos. Nature 246, 313–314. ( 10.1038/246313a0) [DOI] [Google Scholar]
- 22.Hatt RT. 1932. The vertebral columns of ricochetal rodents. Bull. AMNH 63, 599–738. [Google Scholar]
- 23.Essner RL. 2002. Three-dimensional launch kinematics in leaping, parachuting and gliding squirrels. J. Exp. Biol. 205, 2469–2477. [DOI] [PubMed] [Google Scholar]
- 24.Bullen R, McKenzie NL. 2001. Bat airframe design: flight performance, stability, and control in relation to foraging ecology. Aust. J. Zool. 49, 235–261. ( 10.1071/ZO00037) [DOI] [Google Scholar]
- 25.Organ JM, Teaford MF, Taylor AB. 2009. Functional correlates of fiber architecture of the lateral caudal musculature in prehensile and nonprehensile tails of the platyrrhini (primates) and procyonidae (carnivora). Anat. Rec. 292, 827–841. ( 10.1002/ar.20886) [DOI] [PubMed] [Google Scholar]
- 26.Rosenberger AL. 1983. Tale of tails: parallelism and prehensility. Am. J. Phys. Anthropol. 60, 103–107. ( 10.1002/ajpa.1330600114) [DOI] [PubMed] [Google Scholar]
- 27.O'Connor SM, Dawson TJ, Kram R, Donelan JM. 2014. The kangaroo's tail propels and powers pentapedal locomotion. Biol. Lett. 10, 20140381 ( 10.1098/rsbl.2014.0381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton RL. 1972. An experimental study of predatory and feeding behavior in the cheetah (Acinonyx jubatus). Ethology 31, 270–280. ( 10.1111/j.1439-0310.1972.tb01768.x) [DOI] [PubMed] [Google Scholar]
- 29.Wilson AM, Lowe J, Roskilly K, Hudson PE, Golabek K, McNutt J. 2013. Locomotion dynamics of hunting in wild cheetahs. Nature 498, 185 ( 10.1038/nature12295) [DOI] [PubMed] [Google Scholar]
- 30.Patel A, Braae M. 2014. Rapid acceleration and braking: Inspirations from the cheetah's tail. In 2014 IEEE Int. Conf. on Robotics and Automation (ICRA), pp. 793–799, IEEE. [Google Scholar]
- 31.Bennett AF, Huey RB, John-Alder H, Nagy KA. 1984. The parasol tail and thermoregulatory behavior of the Cape ground squirrel Xerus inauris. Physiol. Zool. 57, 57–62. ( 10.1086/physzool.57.1.30155968) [DOI] [Google Scholar]
- 32.Fish FE. 1979. Thermoregulation in the muskrat (Ondatra zibethicus): the use of regional heterothermia. Comp. Biochem. Physiol. 64, 391–397. ( 10.1016/0300-9629(79)90459-6) [DOI] [Google Scholar]
- 33.Muchlinski AE, Shump KA. 1979. The sciurid tail: a possible thermoregulatory mechanism. J. Mamm. 60, 652–654. ( 10.2307/1380118) [DOI] [Google Scholar]
- 34.Stitt JT, Hardy JD. 1971. Thermoregulation in the squirrel monkey (Saimiri sciureus). J. Appl. Physiol. 31, 48–54. ( 10.1152/jappl.1971.31.1.48) [DOI] [PubMed] [Google Scholar]
- 35.Wilson DR. 1972. Tail reduction in Macaca. In The functional and Evolutionary Biology of Primates (ed. Tuttle R.), pp. 241–261. New York, NY: Routledge. [Google Scholar]
- 36.Larson SG, Stern JT Jr. 2006. Maintenance of above-branch balance during primate arboreal quadrupedalism: coordinated use of forearm rotators and tail motion. Am. J. Phys. Anthropol. 129, 71–81. ( 10.1002/ajpa.20236). [DOI] [PubMed] [Google Scholar]
- 37.Rodman PS. 1979. Skeletal differentiation in Macaca fascicularis and Macaca nemestrina in relation to arboreal and terrestrial quadrapedalism. Am. J. Phys. Anthropol. 51, 51–62. ( 10.1002/ajpa.1330510107) [DOI] [Google Scholar]
- 38.Martin RD. 1968. Reproduction and ontogeny in tree-shrews (Tupaia belangeri), with reference to their general behaviour and taxonomic relationships. Ethology 25, 409–495. ( 10.1111/j.1439-0310.1968.tb00026.x) [DOI] [PubMed] [Google Scholar]
- 39.Grand TI. 1984. Motion economy within the canopy: four strategies for mobility: adaptations for foraging in nonhuman primates: contributions to an organismal biology of prosimians. In Monkeys Apes (eds Rodman PS, Cant JGH), pp. 54–72. New York, NY: Colombia University Press; ( 10.7312/rodm90184-004) [DOI] [Google Scholar]
- 40.Organ JM. 2008. The functional anatomy of prehensile and nonprehensile tails of the Platyrrhini (Primates) and Procyonidae (Carnivora). Baltimore, MD: The Johns Hopkins University. [DOI] [PubMed] [Google Scholar]
- 41.Osborn RC. 1908. Adaptations to aquatic, arboreal, fossorial and cursoiral habits in mammals. I. Aquatic adaptations. Am. Nat. 37, 651–665. ( 10.1086/278351) [DOI] [Google Scholar]
- 42.Fish FE. 2016. Secondary evolution of aquatic propulsion in higher vertebrates: validation and prospect. Integr. Comp. Biol. 56, 1285–1297. ( 10.1093/icb/icw123) [DOI] [PubMed] [Google Scholar]
- 43.Lawlor TE. 1973. Aerodynamic characteristics of some neotropical bats. J. Mamm. 54, 71–78. ( 10.2307/1378873) [DOI] [Google Scholar]
- 44.Shargal E, Rath-Wolfson L, Kronfeld N, Dayan T. 1999. Ecological and histological aspects of tail loss in spiny mice (Rodentia: Muridae, Acomys) with a review of its occurrence in rodents. J. Zool. 249, 187–193. ( 10.1111/j.1469-7998.1999.tb00757.x) [DOI] [Google Scholar]
- 45.Hickman GC. 1984. Surface-mound formation by the Tuco-tuco, Ctenomys fulvus (Rodentia: Ctenomyidae), with comments on earth-pushing in other fossorial mammals. J. Zool. 205, 385–390. ( 10.1111/j.1469-7998.1985.tb05624.x) [DOI] [Google Scholar]
- 46.Hildebrand M, Goslow GE, Hildebrand V. 1995. Analysis of vertebrate structure. New York, NY: John Wiley & Sons. [Google Scholar]
- 47.Steen I, Steen J. 1965. Thermoregulatory importance of the beaver's tail. Comp. Biochem. Physiol. 15, 267–270. ( 10.1016/0010-406X(65)90352-X) [DOI] [PubMed] [Google Scholar]
- 48.Walker C, Vierck CJ, Ritz LA. 1998. Balance in the cat: role of the tail and effects of sacrocaudal transection. Behav. Brain Res. 91, 41–47. ( 10.1016/S0166-4328(97)00101-0) [DOI] [PubMed] [Google Scholar]
- 49.Demes B, Jungers WL, Fleagle JG, Wunderlich RE, Richmond BG, Lemelin P. 1996. Body size and leaping kinematics in Malagasy vertical clingers and leapers. J. Hum. Evol. 31, 367–388. ( 10.1006/jhev.1996.0066) [DOI] [Google Scholar]
- 50.Garber PA, Rehg J. 1999. The ecological role of the prehensile tail in white-faced capuchins (Cebus capucinus). Am. J. Phys. Anthropol. 110, 325–339. () [DOI] [PubMed] [Google Scholar]
- 51.Smaers JB, Turner AH, Gómez-Robles A, Sherwood CC. 2018. A cerebellar substrate for cognition evolved multiple times independently in mammals. eLife 7, e35696 ( 10.7554/eLife.35696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Packard GC, Boardman TJ. 1987. The misuse of ratios to scale physiological data that vary allometrically with body size. In New directions in ecological physiology (eds Feder ME, Bennett AF, Burggren WW, Hue RB). vol. 61, pp. 1–9. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 53.Preuschoft H, Witte H, Christian A, Fischer M. 1996. Size influences on primate locomotion and body shape, with special emphasis on the locomotion of ‘small mammals’. Folia Primatol. 66, 93–112. ( 10.1159/000157188) [DOI] [PubMed] [Google Scholar]
- 54.MacKenzie AE, Begun DR. 2009. The ancestor's tail: evolution of taillessness within the catarrhini. Am. J. Phys. Anthropol. 138, 180. [Google Scholar]
- 55.Smaers JB, Rohlf FJ. 2016. Testing species' deviation from allometric predictions using the phylogenetic regression. Evolution 70, 1145–1149. ( 10.1111/evo.12910) [DOI] [PubMed] [Google Scholar]
- 56.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language Bioinformatics 20, 289–290. [DOI] [PubMed] [Google Scholar]
- 57.Pinheiro J, Bates D, DebRoy S, Sarkar D.. 2006. nlme: an R package for fitting and comparing Gaussian linear and nonlinear mixed-effects models. See http://www.stats.bris.ac.uk/R.
- 58.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 59.Uyeda JC, Eastman J, Harmon L. 2013. bayou: Bayesian fitting of Ornstein-Uhlenbeck models to phylogenies. R package version 2.0.
- 60.Adams D, Collyer M, Kaliontzopoulou A, Sherratt E.. 2016. Geomorph: Software for geometric morphometric analyses. R package version 3.0. See http://cran.r-project.org/web/packages/geomorph/index.html.
- 61.Denton JS, Adams DC. 2015. A new phylogenetic test for comparing multiple high-dimensional evolutionary rates suggests interplay of evolutionary rates and modularity in lanternfishes (Myctophiformes; Myctophidae). Evolution 69, 2425–2440. ( 10.1111/evo.12743) [DOI] [PubMed] [Google Scholar]
- 62.Adams DC. 2012. Comparing evolutionary rates for different phenotypic traits on a phylogeny using likelihood. Syst. Biol. 62, 181–192. ( 10.1093/sysbio/sys083) [DOI] [PubMed] [Google Scholar]
- 63.Adams DC. 2014. Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Syst. Biol. 63, 166–177. ( 10.1093/sysbio/syt105) [DOI] [PubMed] [Google Scholar]
- 64.Felsenstein J. 1973. Maximum-likelihood estimation of evolutionary trees from continuous characters. Am. J. Hum. Genet. 25, 471. [PMC free article] [PubMed] [Google Scholar]
- 65.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zool. Scripta 26, 331–348. ( 10.1111/j.1463-6409.1997.tb00423.x). [DOI] [Google Scholar]
- 66.Fleagle JG. 1985. Size and adaptation in primates. In Size and scaling in primate biology (ed. Jungers WJ.), pp. 1–19. Boston, MA: Springer. [Google Scholar]
- 67.Kiley-Worthington M. 1976. The tail movements of ungulates, canids, and felids. Behaviour 56, 69–114. ( 10.1163/156853976X00307) [DOI] [Google Scholar]
- 68.Lemelin P. 1995. Comparative and functional myology of the prehensile tail in New World monkeys. J. Morphol. 224, 351–368. ( 10.1002/jmor.1052240308) [DOI] [PubMed] [Google Scholar]
- 69.Schmitt D, Rose MD, Turnquist JE, Lemelin P. 2005. Role of the prehensile tail during ateline locomotion: experimental and osteological evidence. Am. J. Phys. Anthropol. 126, 435–446. ( 10.1002/ajpa.20075) [DOI] [PubMed] [Google Scholar]
- 70.Lambert TD, Halsey MK. 2015. Relationship between lianas and arboreal mammals: examining the Emmons–Gentry hypothesis. In Ecology of Lianas (eds Schnitzer SA, Bongers F, Burnham RJ, Putz FE), pp. 398–406. Chichester, UK: John Wiley. [Google Scholar]
- 71.Shimer HW. 1903. Adaptations to aquatic, arboreal, fossorial, and cursorial habits in mammals. IV. Fossorial adaptations. Am. Nat. 37, 819–825. ( 10.1086/278368) [DOI] [Google Scholar]
- 72.Alho JS, Herczeg G, Laugen AT, Rasanen K, Laurila A, Merila J. 2011. Allen's rule revisited: quantitative genetics of extremity length in the common frog along a latitudinal gradient. J. Evol. Biol. 24, 59–70. ( 10.1111/j.1420-9101.2010.02141.x) [DOI] [PubMed] [Google Scholar]
- 73.McNab BK. 1966. The metabolism of fossorial rodents: a study of convergence. Ecology 47, 712–733. ( 10.2307/1934259) [DOI] [Google Scholar]
- 74.Stricker EM, Hainsworth FR. 1971. Evaporative cooling in the rat: interaction with heat loss from the tail. Exp. Physiol. 56, 231–241. ( 10.1113/expphysiol.1971.sp002124) [DOI] [PubMed] [Google Scholar]
- 75.Tilkens MJ, Wall-Scheffler C, Weaver TD, Steudel-Numbers K. 2007. The effects of body proportions on thermoregulation: an experimental assessment of Allen's rule. J. Hum. Evol. 53, 286–291. ( 10.1016/j.jhevol.2007.04.005) [DOI] [PubMed] [Google Scholar]
- 76.Serrat MA, King D, Lovejoy CO. 2008. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc. Natl Acad. Sci. USA 105, 19 348–19 353. ( 10.1073/pnas.0803319105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ceballos G, Ehrlich PR. 2006. Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl Acad. Sci. USA 103, 19 374–19 379. ( 10.1073/pnas.0609334103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleagle JG, Lieberman DE. 2015. Major transformations in the evolution of primate locomotion. In Great transformations in vertebrate evolution (eds Dial KP, Shubin NH, Brainerd EL), pp. 257–278. Chicago, IL: University of Chicago Press. [Google Scholar]
- 79.Fleagle JG. 1984. Primate locomotion and diet. In Food acquisition and processing in primates (eds Chivers DJ, Wood BA, Bilsborough A), pp. 105–117. Boston, MA: Springer. [Google Scholar]
- 80.Sehner S, Fichtel C, Kappeler PM. 2018. Primate tails: ancestral state reconstruction and determinants of interspecific variation in primate tail length. Am. J. Phys. Anthropol. 167, 750–759. ( 10.1002/ajpa.23703) [DOI] [PubMed] [Google Scholar]
- 81.Hamada Y, Yamamoto A, Kunimatsu Y, Tojima S, Mouri T, Kawamoto Y. 2012. Variability of tail length in hybrids of the Japanese macaque (Macaca fuscata) and the Taiwanese macaque (Macaca cyclopis). Primates 53, 397–411. ( 10.1007/s10329-012-0317-3) [DOI] [PubMed] [Google Scholar]
- 82.Roos C, Zinner D. 2015. Diversity and evolutionary history of macaques with special focus on Macaca mulatta and Macaca fascicularis. In The nonhuman primate in nonclinical drug development and safety assessment (eds Bluemel J, Korte S, Schenk E, Weinbauer G), pp. 3–16. Elsevier. [Google Scholar]
- 83.Fooden J. 2006. Comparative review of fascicularis-group species of macaques (Primates: Macaca). Fieldiana Zool. 107, 1–43. ( 10.5962/bhl.title.3580) [DOI] [Google Scholar]
- 84.Wakamori H, Hamada Y. 2019. Skeletal determinants of tail length are different between macaque species groups. Sci. Rep. 9, 1289 ( 10.1038/s41598-018-37963-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Preuschoft H. 2002. What does ‘arboreal locomotion’ mean exactly and what are the relationships between ‘climbing’, environment and morphology? Zeitschrift für Morphologie und Anthropologie 83, 171–188. [PubMed] [Google Scholar]
- 86.McNulty KP, Begun DR, Kelley J, Manthi FK, Mbua EN. 2015. A systematic revision of Proconsul with the description of a new genus of early Miocene hominoid. J. Hum. Evol. 84, 42–61. ( 10.1016/j.jhevol.2015.03.009) [DOI] [PubMed] [Google Scholar]
- 87.Ward CV. 1993. Torso morphology and locomotion in Proconsul nyanzae. Am. J. Phys. Anthropol. 92, 291–328. ( 10.1002/ajpa.1330920306) [DOI] [PubMed] [Google Scholar]
- 88.Deinard A, Smith DG. 2001. Phylogenetic relationships among the macaques: evidence from the nuclear locus NRAMP1. J. Hum. Evol. 41, 45–59. ( 10.1006/jhev.2001.0480) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.