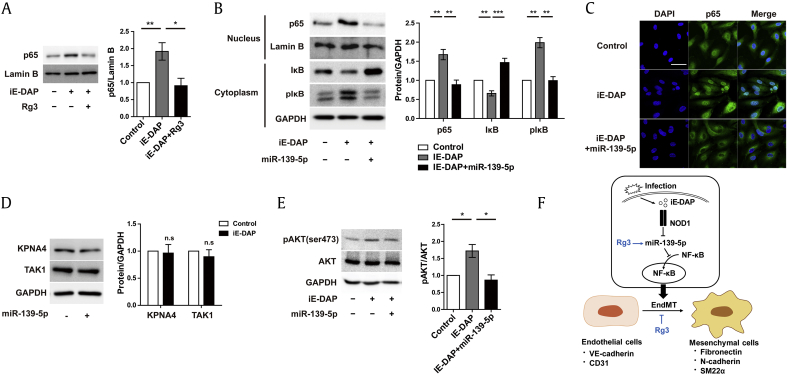
Fig. 4.
Rg3 suppresses iE-DAP–induced EndMT via Akt/NF-κB signaling. (A) Nuclear p65 protein expression in response to iE-DAP (20 μg/mL) with or without concurrent treatment with Rg3 (10 μg/mL) for 2 d. (B) Protein expression of nuclear p65, cytosolic IκB, and cytosolic p-IκB in response to iE-DAP (20 μg/mL) with or without miR-139-5p overexpression (12 nM) for 2 d. (C) Immunostaining images of nuclear p65 translocation in response to iE-DAP (20 μg/mL) with or without miR-139-5p overexpression (12 nM) for 2 d. Scale bar = 50 μm. (D) Importin-α3 and TAK1 protein expression in response to lentiviral miR-139-5p overexpression for 4 d. (E) Protein expression of p-Akt and Akt in response to iE-DAP (20 μg/mL) with or without miR-139-5p overexpression (12 nM) for 2 d. (F) Schematic outlining of the proposed mechanism in ECs. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the controls, as determined by the unpaired two-tailed Student t test. Error bars, s.e.m. N = 3 experiments per condition.
ECs, endothelial cells; EndMT, endothelial-to-mesenchymal transition; iE-DAP, γ-d-glutamyl-meso-diaminopimelic acid; s.e.m., standard error of the mean; Rg3, ginsenoside Rg3; TAK1, transforming growth factor beta-activating kinase 1.