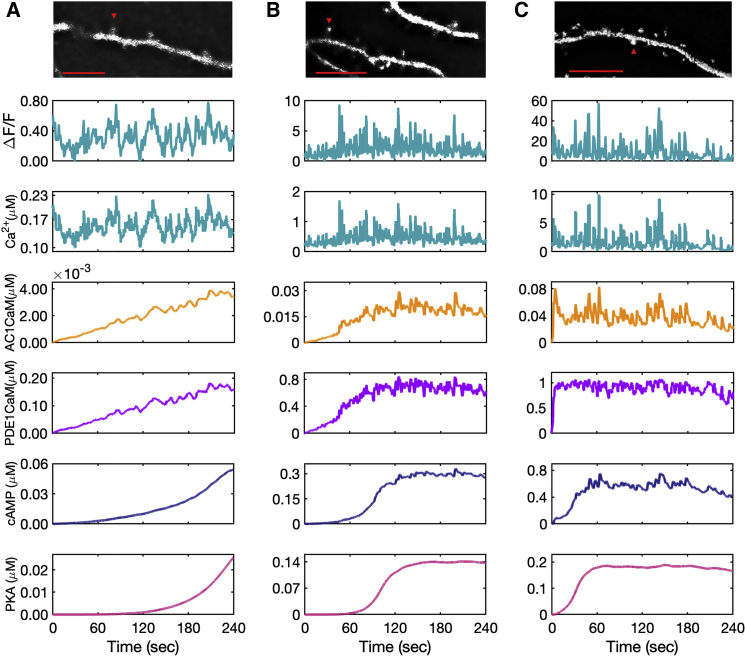
Figure 6.
Model predictions for AC1 ⋅ CaM, PDE1 ⋅ CaM, cAMP, and PKA for experimental calcium input measured from GCaMP6f expressed in DIV 22 primary rat hippocampal neurons. Fluorescence intensity measurements shown for calcium have an arbitrary starting time, and the model system at time zero (start of the recording) is already in equilibrium. The calcium concentration is estimated based on the relative increases and decreases in calcium to the minimum intensity measured. (A) The calcium input is at a low range, almost on the range of resting calcium concentration. This calcium amount slows down the rate of AC1 and PDE1 activation by the calcium-calmodulin complex, and as a result, only a low amount of cAMP is produced. AC1 ⋅ CaM and PDE1 ⋅ CaM seem to pick up only calcium peaks, whereas these calcium dynamics seem to barely affect the cAMP and PKA dynamics over the time course of recorded calcium (4 min). (B) The second set of measured calcium in the spine shows a higher amount of calcium, which accelerates the rate of AC1 and PDE1 activation and cAMP and PKA formation. Because of the higher range of calcium oscillations in this data set, AC1 ⋅ CaM, PDE1 ⋅ CaM, and cAMP seem to be affected more by calcium transients. (C) This set of measured calcium data shows the highest recorded calcium. Because of the high range of calcium oscillations (10 μM), the induced calcium transients are significant in this case, and even PKA shows a subtle effect of the most prominent calcium spikes. The data are representative of n = 10 neurons from three independent experiments. Representative images at the indicated time points are shown for each spine selected for analysis. Scale bars, 10 μm. To see this figure in color, go online.