Highlights
-
•
The associations between metabolic syndrome and physical activity were compared between healthy middle-aged American men and Japanese men.
-
•
Higher total step counts/day had a protective effect on metabolic syndrome in both cohorts.
-
•
The effect of steps/day was greater than domain-specific moderate-vigorous physical activity captured by questionnaire.
-
•
Tools that capture total movement may be important to assess the effects of physical activity on metabolic syndrome.
Keywords: Epidemiology, Metabolic syndrome, Pedometer, Physical activity, Questionnaire
Abstract
Background
Metabolic syndrome (MetS) is a global health problem. Physical activity (PA) is a known modifiable risk factor for MetS and individual MetS components. However, the role of PA could differ between sub-populations due to differences in the variability of PA and other MetS risk factors. To examine these differences, multi-country studies with standardized outcome measurement methods across cohorts are needed.
Methods
Cross-sectional PA levels (total and domain specific) in healthy middle-aged (44–56 years) men in the Risk Factor Assessment among Japanese and U.S. Men in the Post-World War II Birth Cohort (ERA-JUMP) Study (n = 730; American: n = 417; Japanese: n = 313; from population-representative samples in Pittsburgh, Pennsylvania, USA, and Kusatsu, Shiga, Japan) were compared. The relationships between PA levels and MetS (overall and specific components) in/across the American and Japanese sub-cohorts (adjusting for age, smoking, and alcohol consumption) were also assessed using the same instruments (pedometer and validated questionnaire) to measure PA in both cohorts.
Results
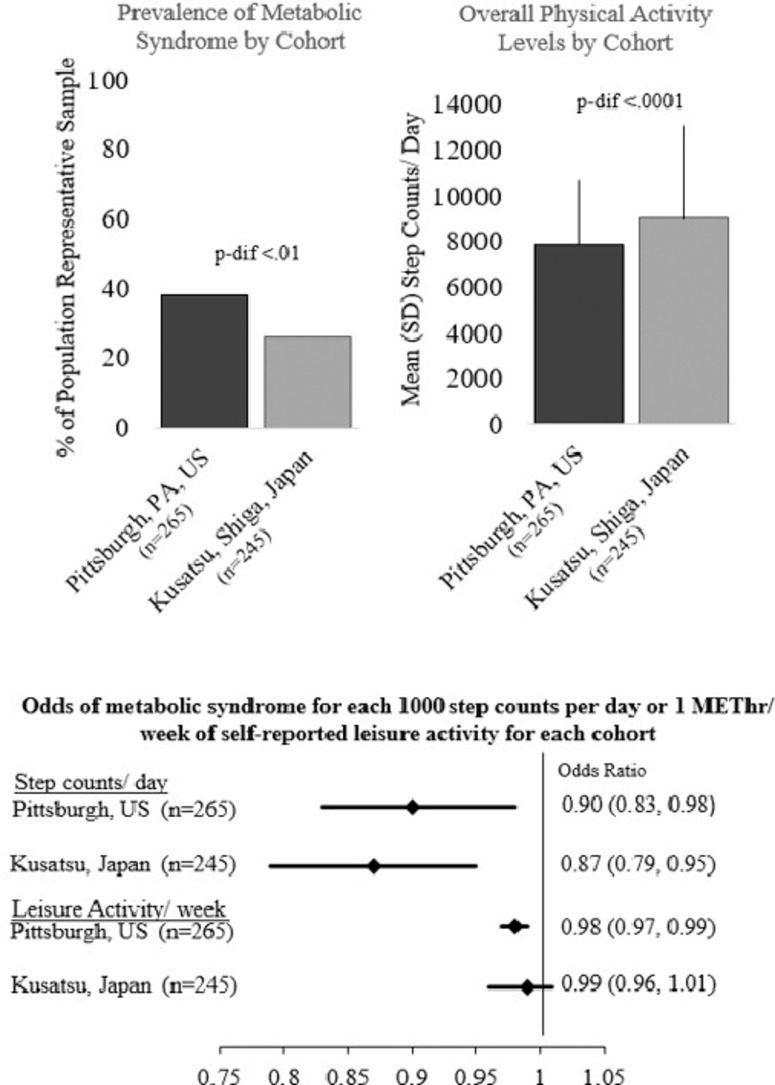
A total of 510 individuals provided complete data on PA (American: n = 265; Japanese: n = 245). The American cohort had significantly lower mean ± SD steps/day (7878 ± 3399 steps/day) vs. the Japanese cohort (9055 ± 3797 steps/day) (p < 0.001) but had significantly higher self-reported moderate-vigorous leisure PA (American: 15.9 (7.4–30.3) metabolic task equivalent hours per week (MET-h/week) vs. Japanese: 4.0 (0–11.3) MET-h/week, p < 0.0001). In both sub-cohorts, each 1000 steps/day increase was associated with lower odds of having MetS (American: OR = 0.90, 95%CI: 0.83–0.98; Japanese: OR = 0.87, 95%CI: 0.79–0.95) and the individual MetS component of high waist circumference (American: OR = 0.86, 95%CI: 0.79–0.94; Japanese: OR = 0.87, 95%CI: 0.80–0.95). In the American cohort only, higher self-reported leisure PA (Met-h/week) was associated with lower odds of MetS and high waist circumference (OR = 0.98, 95%CI: 0.97–0.99 for MetS and waist circumference, respectively).
Conclusion
Higher total step counts/day had an important protective effect on MetS prevalence in both the Japanese and American cohorts, despite differences in PA levels and other MetS risk factors. The effect of steps/day (across all intensity levels) was much greater than domain-specific moderate-vigorous PA captured by questionnaire, suggesting the need for measurement tools that can best capture total movement when examining the effects of PA on MetS development.
Graphical abstract
1. Introduction
Metabolic syndrome (MetS), a cluster of metabolic risk factors leading to the development of cardiovascular disease and diabetes, is a rapidly increasing global threat, affecting 20%–30% of adults worldwide.1 It is expected to increase further, given aging populations in many countries and changes in lifestyle habits related to westernization.1, 2, 3
Physical activity (PA) is an important modifiable factor for MetS that is available to people around the world.4 Studies in various countries have shown the associations between MetS and PA in their population cohorts.5, 6, 7, 8 However, there is variability across populations in regard to how much PA contributes to MetS prevalence.5, 6, 7, 8 Without consistency in the measurement methods for PA, it is currently unknown whether these perceived differences in risk contribution across different cultures or countries are real or are artifacts of differential bias caused by using different methods of PA measurement.9 This is unfortunate because examining the associations between PA levels and MetS across cohorts with different lifestyle habits and different rates of MetS has the potential to better illuminate the mechanisms underlying the relationship between PA and MetS.
Most previous studies have used recall questionnaires to assess self-reported PA levels.5, 6, 7, 8 However, studies with cohorts from more than 1 country are rare and there are many different types of PA questionnaires, resulting in the use of different questionnaires across studies. In general, questionnaires are best for measuring domain-specific planned activities (e.g., leisure, occupational, and commuting) that are of moderate-to-vigorous intensity because people are able to recall these activities better.10 Not all questionnaires capture all domains; nevertheless, most studies present their findings as being generically based on “physical activity” regardless of the components of PA that are represented. Even in cases where the same questionnaire is used and translated into different languages, there can be problems related to interpretation bias, cultural sensitivity for reporting certain activities, or missing information on activities that are important in one culture versus another.11,12 These issues can make it difficult to interpret differences in the associations between PA and MetS in cohorts from different countries because perceived differences in risk association could simply result from differences in how PA is measured and which components of PA are reported across questionnaires.
Some recent studies using objective measures such as pedometers or accelerometers have greater potential for comparability across studies because pedometers and accelerometers are not subject to the same types of reporting bias as recall questionnaires and allow for a higher degree of standardization of measurement across populations when the same instrument, protocol, and processing methods are utilized.13 These monitors primarily capture ambulatory movement. Although they may misreport (or not report) non-ambulatory activities, such as biking or swimming, the output from these devices is often used as a proxy for total movement, which is something that cannot be validly assessed by questionnaire.10,13 However, because pedometers and accelerometers cannot capture domain-specific PA, such as leisure PA (which is typically the target of PA intervention programs), it has been suggested that the most effective method of measuring PA is with the combination of accelerometers or pedometers and a questionnaire. Thus, to provide the best assessment of the relationship between PA and MetS across countries, both a questionnaire that reports domain-specific PA and can be modified to suit all included populations and an objective measure of total movement are needed.14
There have been only a few studies comparing PA across countries, and all of them used different questionnaire-based methods for the assessment of PA without using pedometers or accelerometers.15, 16, 17, 18 This is unfortunate because examining the associations between PA levels and MetS across cultures with different lifestyle habits and different rates of MetS has the potential to better illuminate the mechanisms underlying the relationship between PA and MetS and to provide better international PA guidelines geared toward lowering the prevalence of MetS and the development of lifestyle-induced cardiovascular disease and diabetes.
The United States has one of the highest rates of MetS, affecting about 30% of adults,19 whereas only 5%–10% of Japanese adults have MetS,20 which is one of the lowest rates in the world. Additionally, 1 study based on self-reported PA suggested that Americans may perform PA at more vigorous levels while Japanese engage in more moderate-intensity walking.18 However, no previous studies have uniformly collected and directly compared PA levels in the United States and Japan using objective methods, and no previous studies have compared the association of PA and MetS across these 2 countries. Therefore, examining differences in risk contribution of both total PA and domain-specific PA from standardized and validated instruments across cohorts in Japan and the United States has the potential to contribute new knowledge on the importance of PA as a modifiable risk factor for MetS prevalence in both countries. The results of such a study may also help to inform future international efforts on how to measure the contribution of PA to the development of MetS and individual MetS components.
Using data from the electron beam computed tomography and Risk Factor Assessment among Japanese and U.S. Men in the Post-World War II Birth Cohort (ERA-JUMP) Study, we compared characteristics of PA across the American and Japanese cohorts. We also examined the associations between both subjectively and objectively measured PA levels and MetS in each cohort using a single questionnaire and standardized collection/evaluation tools for pedometer step counts across cohorts.
2. Materials and methods
2.1. Study population
Details of the ERA-JUMP Study have been described previously.21,22 Briefly, the ERA-JUMP Study was established to investigate differences in subclinical atherosclerosis among men aged 40–49 years at baseline (2002–2006) in different populations. Study participants were population-based samples of American men from Allegheny County, Pennsylvania, USA, and Japanese men from Kusatsu, Shiga, Japan, all of whom completed a follow-up examination between 2008 and 2013 (when PA assessments were completed for both the American and Japanese sub-cohorts in the ERA-JUMP Study). Both the American (n = 417) and Japanese (n = 313) cohorts were randomly selected from local registries. All participants were without clinical cardiovascular disease, type 1 diabetes, or other severe disease. The current study utilized cross-sectional data from the follow-up visit for the main ERA-JUMP Study because that is when PA data from questionnaires and pedometers were collected from both cohorts.
We excluded 172 participants who did not have a follow-up visit (114 American men and 58 Japanese men) and 48 participants who did not have pedometer and/or modifiable activity questionnaire (MAQ) data (38 American men and 10 Japanese men). Our final sample consisted of 510 participants: 265 American (222 Caucasian and 43 African American) men and 245 Japanese men. Compared to our final sample, the 172 participants who were originally excluded because they did not have a follow-up visit had a significantly higher body mass index (BMI) (American: 26.1 ± 4.6 vs. Japanese: 27.0 ± 5.7, p = 0.048), although no differences existed for age, prevalence of MetS and its components, or percentage with self-reported leisure PA of 1 h/week or more (data not shown). The present study was approved by the Institutional Review Boards of the University of Pittsburgh and Shiga University of Medical Science and written informed consent was collected from all participants.
2.2. PA assessment
To capture both total and domain-specific PA (leisure and occupational), both subjective and objective measurement tools were used. The MAQ, a previously validated interviewer-administered recall questionnaire, was used to assess both leisure and occupational activities that mostly were of moderate-to-vigorous intensity.23,24 The MAQ version used in this study was specifically modified for use across these 2 country-specific cohorts and has previously been modified and translated for use in several different countries. Prior to administration, the MAQ was piloted at both study sites to ensure the comprehensive inclusion of activities that were culturally relevant for both sites.25 All staff administering the MAQ were trained and certified prior to data collection. Participants were asked to report primarily moderate- to vigorous-intensity leisure and occupational activity that occurred during the 6 months immediately before their clinic visit. PA levels were then calculated by the staff as the product of the duration and frequency of each activity (in h/week), weighted by the estimate of the metabolic task equivalent (MET)26 of that activity (from the Compendium of Physical Activities),27 and summed for all activities performed. Data were expressed as MET hours per week (MET-h/week) averaged over the 6-month period.
Objectively measured PA data were collected via the Yamax Digiwalker SW-200 pedometer (Yamasa Tokei Keiki Corporation, Tokyo, Japan). The digiwalker SW-200 is a spring-levered pedometer that records steps taken. Pedometers are best at assessing ambulatory activity levels and collect both moderate- to vigorous-intensity and light-intensity movement. The validity and reliability of this device in counting steps have been shown in various laboratory and field settings.28,29
Participants were asked to wear the pedometer during all waking hours for 7 consecutive days and to only remove the monitor for sleeping, showering, and other water activities. Participants recorded the number of steps taken in an activity diary at the end of each day. They were also asked to report monitor time on/off. Monitors and diaries were then returned to the clinic in a postage-paid envelope. Per previously validated protocol, only participants with 3 or more days of data were included in the study.30 If there was a question regarding compliance with monitor wear instructions, diary on/off times were used to confirm compliance. The data were then summed as average step counts per day (the sum of total step counts recorded divided by the number of reported days). Pedometer steps were further categorized to correspond with previously reported cut points for low (<5000 steps/day), moderate (5000 to <10,000 steps/day), and high (≥10,000 steps/day) activity.31
2.3. Other measures
Body weight and height were measured with the participants wearing light clothing but without shoes. BMI was calculated as weight (in kilograms) divided by height squared (in meters squared). Waist circumference (WC) was measured with a tape measure at the level of the umbilicus while the participants were standing and at the end of exhalation. Blood pressure (BP) was measured in the right arm of the seated participants after emptying their bladder and sitting quietly for 5 min, using an automated sphygmomanometer (BP-8800; Colin Medical Technology, Komaki, Japan). The average of 2 measurements was used in the analyses. Smoking (never, former, or current), alcohol consumption (≥2 days/week), and medication use (anti-hypertensive, anti-diabetic, and lipid lowering) was assessed via questionnaire. Venipuncture was performed early in the clinic visit after a 12-h fast. Serum and plasma samples were stored at –80°C and shipped to the University of Pittsburgh. Serum/plasma samples were assayed for glucose and lipids as described previously.21 For the comparison of BP, blood glucose, and lipid levels, those on medications were excluded. MetS and individual components were defined according to the updated National Cholesterol Education Program Adult Treatment Panel-III criteria, which take into account medications for the different components.32 Specifically, MetS was defined as 3 or more of 5 components: elevated WC (≥102 cm in American men and ≥90 cm in Japanese men), elevated BP (systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg), elevated triglycerides (≥150 mg/dL), reduced high-density lipoprotein (HDL) cholesterol (<40 mg/dL), and elevated fasting glucose (≥100 mg/dL). Per protocol, individuals on BP, blood glucose, and/or lipid-lowering medications were defined as having the treated MetS components. However, a sensitivity analysis was also done in which individuals on medications were not included. Obesity was defined as a BMI of 25 kg/m2or greater for Japanese men and BMI of 30 kg/m2or greater for American men.33
2.4. Statistical analysis
Means, medians, and frequencies of characteristics between the 2 populations were compared using the t test, Wilcoxon rank-sum test, and χ2 test, as appropriate. Specifically, fasting glucose and triglycerides were compared using the Wilcoxon rank-sum test. American men were further classified by race (Caucasian and African American) to describe characteristics of participants. Odds ratios (ORs) for having MetS, MetS components, and obesity per 1000 pedometer steps and separately, per 1 MET-h/week from the MAQ (equivalent to 20 min of moderate-intensity PA at 3.0 METs) were assessed using logistic regression analysis adjusted for important covariates, including age, smoking history, and alcohol consumption. Statistical significance was determined by a p value of less than 0.05. All statistical analyses were performed using SAS software Version 9.4 (SAS Institutes Inc., Cary, NC, USA).
3. Results
3.1. Characteristics of participants
At the follow-up visit, American participants (n = 265) appeared to be slightly younger than Japanese participants (n = 245) on average (49.8 ± 2.9 years vs. 51.2 ± 2.8 years, p < 0.0001) (Table 1). American participants also had a higher BMI than their Japanese counterparts (29.2 ± 4.9 kg/m2 vs. 23.8 ± 3.2 kg/m2, p < 0.0001), although no difference in obesity was observed after using ethnic-group-specific BMI cut points (32% vs. 31%, p = 0.70). A significantly higher percentage of Japanese men reported past or current smoking and alcohol consumption ≥2 times/week (both p < 0.0001) (Table 1).
Table 1.
Characteristics of participants by site (men aged 44–56 years, 2008–2013).
| Pittsburgh (n = 265) | Kusatsu (n = 245) | p | |
|---|---|---|---|
| Age (year) | 49.8 ± 2.9 | 51.2 ± 2.8 | <0.0001 |
| Education (year) | 16.8 ± 2.8 | 14.3 ± 2.1 | <0.0001 |
| Smoking status | <0.0001 | ||
| Never | 194 (73) | 46 (19) | |
| Past | 49 (18) | 103 (42) | |
| Current | 22 (8) | 96 (39) | |
| Alcohol (≥2 times/week) | 116 (44) | 164 (67) | <0.0001 |
| Comorbidity | |||
| Waist circumference (cm) | 101.8 ± 13.1 | 85.7 ± 8.7 | <0.0001 |
| BMI (kg/m2) | 29.2 ± 4.9 | 23.8 ± 3.2 | <0.0001 |
| Obesitya | 85 (32) | 75 (31) | 0.70 |
| Systolic blood pressure (mmHg)b | 126.2 ± 13.3 | 126.9 ± 16.0 | 0.59 |
| Diastolic blood pressure (mmHg)b | 76.5 ± 8.8 | 80.5 ± 11.5 | <0.0001 |
| Antihypertensive mediations | 51 (19) | 39 (16) | 0.34 |
| Hypoglycemic medications | 12 (5) | 12 (5) | 0.84 |
| Lipid-lowering medications | 54 (21) | 17 (7) | <0.0001 |
| Total cholesterol (mg/dL)b | 217.9 ± 38.0 | 211.4 ± 33.9 | 0.06 |
| HDL cholesterol (mg/dL)b | 50.0 ± 12.3 | 56.5 ± 16.7 | <0.0001 |
| LDL cholesterol (mg/dL)b | 137.9 ± 33.6 | 126.6 ± 32.4 | <0.001 |
| Triglycerides (mg/dL)b | 148.9 ± 85.0 | 144.9 ± 110.1 | 0.34 |
| Fasting glucose (mg/dL)b | 107.0 ± 16.1 | 101.8 ± 11.3 | <0.0001 |
| Metabolic syndromec | 102 (38) | 63 (26) | 0.002 |
| High waist circumferenced | 114 (43) | 68 (28) | <0.001 |
| High blood pressuree | 103 (39) | 108 (44) | 0.23 |
| High glucosef | 193 (73) | 134 (55) | <0.0001 |
| High triglyceridesg | 101 (38) | 89 (36) | 0.68 |
| Low HDL cholesterolh | 57 (22) | 21 (9) | <0.0001 |
Notes: The bolding was used to highlight metabolic syndrome, which is the primary outcome of interest. Data are mean ± SD or n (%).
a BMI ≥30 kg/m2 in American men and BMI ≥25 kg/m2 in Japanese men; b Analyzed among those without medication use; c ≥3 of 5 components; d ≥102 cm in American men and ≥90 cm in Japanese men; e Systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on medication; f Fasting glucose ≥100 mg/dL or on medication; g Triglycerides ≥150 mg/dL or on medication; h HDL cholesterol <40 mg/dL or on medication.
Abbreviations: BMI = body mass index; HDL = high density lipoprotein; LDL = low density lipoprotein.
Overall, American participants were significantly more likely to have MetS (38% vs. 26%, p = 0.002). Differences in hypoglycemic and antihypertension medication use were small and non-significant between the Japanese and American cohorts (p > 0.05). However, the use of lipid-lowering medications was 3 times higher in the American men when compared to the Japanese men (21% vs. 7%, p < 0.0001). Among other MetS criteria, American participants were also significantly more likely to have high WC, high blood glucose, and low HDL cholesterol, although there was no significant difference in the proportion in each cohort with high BP and high triglycerides (based on p < 0.05). When examining only those without medication, American participants had significantly lower diastolic BP, HDL cholesterol, and higher blood glucose levels (Table 1).
3.2. PA levels
American participants had significantly lower mean (±SD) pedometer steps per day compared to Japanese participants (7878 ± 3399 steps/day vs. 9055 ± 3797 steps/day, p < 0.001). There were significantly fewer American participants with 10,000 or more steps/day (21% vs. 35%, p < 0.001) and significantly more American participants with fewer than 5000 steps/day compared to Japanese participants (18% vs. 11%, p = 0.02) (Table 2).
Table 2.
Physical activity by site.
| Pittsburgh (n = 265) | Kusatsu (n = 245) | p | |
|---|---|---|---|
| Pedometer steps/day | 7878 ± 3399 | 9055 ± 3797 | <0.001 |
| ≥10,000 steps/day | 56 (21) | 86 (35) | <0.001 |
| <5000 steps/day | 48 (18) | 27 (11) | 0.02 |
| Leisure MET-h/week | 15.9 (7.4–30.3) | 4.0 (0–11.3) | <0.0001 |
| ≥7.5 Leisure MET-h/week | 196 (74) | 86 (35) | <0.0001 |
| Occupational MET-h/week | 0 (0–18.5) | 0 (0–110.8) | <0.0001 |
| Total MET-h/week | 25.1 (11.5–63.0) | 25.8 (2.1–123.5) | 0.437 |
| Moderate-vigorous leisure PA ≥1 h/week | 206 (78) | 76 (31) | <0.0001 |
Note: Values are mean ± SD, n (%), or median (IQR).
Abbreviations: IQR = interquartile range; MET = metabolic equivalent task; PA = physical activity
The MAQ past 6-month recall questionnaire was also used to provide information on self-reported PA levels in both cohorts. The top 3 activities in the American cohort were gardening, walking for exercise, and strength training, whereas the top 3 activities in the Japanese cohort were bicycling, walking for exercise, and jogging (Table 3).
Table 3.
Common activity ranking in each site (n (%)).
| Pittsburgh (n = 265) | Kusatsu (n = 245) | |
|---|---|---|
| Leisure activity | ||
| Rank 1 | Gardening 158 (59.6) | Bicycling 66 (26.9) |
| Rank 2 | Walking 153 (57.7) | Walking 59 (24.1) |
| Rank 3 | Strength training 97 (36.6) | Jogging 37 (15.1) |
| Rank 4 | Jogging 82 (30.9) | Calisthenics/ toning 32 (13.1) |
| Rank 5 | Bicycling 81(30.6) | Golf 28 (11.4) |
| Rank 6 | Golf 56 (21.1) | Gardening 25 (10.2) |
| Rank 7 | Stair master 54 (20.4) | Fishing 17 (6.9) |
| Rank 8 | Swimming 30 (11.3)a | Swimming 11 (4.5)a |
| Rank 8 | Basketball 30 (11.3)a | Bowling 11 (4.5)a |
| Rank 10 | Hiking 22 (8.3) | Tennis 10 (4.1) |
| Occupational activity | ||
| Rank 1 | Office worker 195 (73.6) | Office worker 128 (52.2) |
| Rank 2 | Non-office worker 79 (29.8) | Non-office worker 117 (47.8) |
| Rank 3 | Unemployed 11 (4.2) | Unemployed 2 (0.8) |
a Activities with tied ranking.
Overall, American participants had significantly higher self-reported median (interquartile range (IQR)) moderate-vigorous leisure activity levels (15.9 (7.4–30.3) MET-h/week vs. 4.0 (0–11.3) MET-h/week, p < 0.0001) and were more than twice as likely to self-report 1 h or more of moderate-vigorous leisure PA/week (78% vs. 31%). Additionally, the proportion of participants achieving 7.5 or more MET-h/week of leisure activity (equivalent to meeting U.S. and WHO aerobic activity guidelines of 150 min/week of moderate-vigorous intensity PA) was higher in American participants (74% vs. 35%, p < 0.0001) (Table 2).
Conversely, there were fewer American participants reporting any occupational activity in the American cohort (25.7% vs. 44.1 %; data not shown) and the overall distribution of occupational activity levels suggested lower levels for the American participants compared to Japanese participants (median (IQR): 0 (0–18.5) vs. 0 (0–110.8) MET-h/week, p < 0.0001). However, when including only those individuals reporting occupational activity, there was no difference in the median (IQR) MET-h/week of work-related activity (American (n = 68): 118.8 (80.4–165.7) vs. Japanese (n = 108): 120.6 (92.3–155.1) MET-h/week, p = 0.665.) It should be noted that when leisure and occupational PA were summed and reported as “total PA” there was no significant difference in median total activity values between the cohorts (American: 25.1 (11.5–63.0) vs. Japanese: 25.8 (2.1–123.5) MET-h/week, p = 0.437). However, when the American cohort was further classified by race/ethnicity, African American participants reported the highest median total, leisure, and occupational PA levels when compared to both American Caucasians and the Japanese cohort (data not shown).
3.3. PA and MetS
Higher counts of pedometer steps were associated with significantly lower odds of having MetS in both populations (Table 4). This association remained even after adjustment for age, smoking, and alcohol consumption (Table 4). In the fully adjusted model, each 1000 step count/day increase was associated with 10% (OR = 0.90, 95%CI: 0.83–0.98) and 13% (OR = 0.87, 95%CI: 0.80–0.95) lower odds of having MetS among American and Japanese participants, respectively (Table 4). For individual MetS components, higher step counts/day were associated with lower odds of having high WC in both populations (American: OR = 0.86, 95%CI: 0.79–0.94; Japanese: OR = 0.88, 95%CI: 0.80–0.95) and high triglycerides in Japanese participants (OR = 0.93, 95%CI: 0.86–0.99). Although no significant associations were observed for high BP, high glucose, and low HDL cholesterol in either cohort (Table 4). In the fully adjusted model each 1000 steps count/day increase was also associated with lower odds of obesity, which was significant in the American cohort (OR = 0.89, 95%CI: 0.81–0.97), but not the Japanese cohort (OR = 0.93, 95%CI: 0.86–1.01) (Table 4). The results were consistent when pedometer steps were represented as a categorical variable (Table 5).
Table 4.
Association of average pedometer steps/day with metabolic syndrome and its components.
| Pittsburgh (n = 265) |
Kusatsu (n = 245) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||
| β | OR (95%CI) | β | OR (95%CI) | β | OR (95%CI) | β | OR (95%CI) | |
| Metabolic syndrome a | –0.108 | 0.90 (0.83–0.98)* | –0.106 | 0.90 (0.83–0.98)* | –0.1421 | 0.87 (0.79–0.95)* | –0.139 | 0.87 (0.80–0.95)* |
| High waist circumference b | –0.150 | 0.86 (0.79–0.94)* | –0.148 | 0.86 (0.79–0.94)* | –0.1332 | 0.88 (0.80–0.95)* | –0.139 | 0.87 (0.80–0.95)* |
| High triglycerides c | –0.074 | 0.93 (0.86–1.01) | –0.077 | 0.93 (0.86–1.00) | –0.0778 | 0.93 (0.86–0.99)* | –0.078 | 0.93 (0.86–0.99)* |
| High blood pressured | –0.038 | 0.96 (0.89–1.04) | –0.042 | 0.96 (0.90–1.03) | –0.0543 | 0.95 (0.88–1.01) | –0.060 | 0.94 (0.88–1.01) |
| High blood glucose e | –0.036 | 0.97 (0.89–1.04) | –0.033 | 0.97 (0.89–1.05) | –0.0312 | 0.97 (0.91–1.04) | –0.030 | 0.97 (0.91–1.04) |
| Low HDL cholesterol f | –0.003 | 1.00 (0.92–1.09) | –0.006 | 0.99 (0.91–1.08) | –0.0936 | 0.91 (0.80–1.04) | –0.067 | 0.94 (0.82–1.07) |
| Obesity g | –0.123 | 0.89 (0.81–0.97)* | –0.121 | 0.89 (0.81–0.97)* | –0.0642 | 0.94 (0.87–1.01) | –0.071 | 0.93 (0.86–1.01) |
Note: Betas and ORs are expressed per 1000 pedometer steps/day. Model 1: unadjusted; Model 2: Model 1 + age, smoking, and alcohol.
a ≥3 of 5 components; b ≥102 cm in American men and ≥90 cm in Japanese men; c Systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on medication; d Triglycerides ≥150 mg/dL or on medication; e HDL-cholesterol <40 mg/dL or on medication; f Fasting glucose ≥100 mg/dL or on medication; g BMI ≥30 kg/m2 in American men and BMI ≥25 kg/m2 in Japanese men.
* p < 0.05.
Abbreviations: BMI = body mass index; CI = confidential interval; HDL = high density lipoprotein; LDL = low density lipoprotein; OR = odds ratio.
Table 5.
Association of categories of pedometer steps with metabolic syndrome and its components.
| Pittsburgh (n = 265) |
Kusatsu (n = 245) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||||
| <5000 (n = 48) | 5000–9999 (n = 161) | ≥10,000 (n = 56) | <5000 (n = 48) | 5000–9999 (n = 161) | ≥10,000 (n = 56) | <5000 (n = 27) | 5000–9999 (n = 132) | ≥10,000 (n = 86) | <5000 (n = 27) | 5000–9999 (n = 132) | ≥10,000 (n = 86) | |
| Metabolic syndrome a | Ref | 0.99 (0.20–1.89) | 0.31 (0.13–0.75)* | Ref | 0.99 (0.51–1.92) | 0.32 (0.13–0.78)* | Ref | 0.32 (0.14–0.75)* | 0.20 (0.08–0.50)* | Ref | 0.32 (0.14–0.77)* | 0.19 (0.07–0.49)* |
| High waist circumference b | Ref | 0.68 (0.35–1.30) | 0.21 (0.09–0.50)* | Ref | 0.68 (0.35–1.30) | 0.22 (0.09–0.52)* | Ref | 0.58 (0.25–1.35) | 0.24 (0.09–0.63)* | Ref | 0.57 (0.24–1.34) | 0.22 (0.08–0.59)* |
| High triglycerides c | Ref | 1.40 (0.72–2.74) | 0.61 (0.26–1.42) | Ref | 1.42 (0.72–2.79) | 0.60 (0.26–1.41) | Ref | 0.43 (0.19–0.99)* | 0.39 (0.16–0.93)* | Ref | 0.43 (0.19–1.00) | 0.39 (0.16–0.95)* |
| High blood pressured | Ref | 0.68 (0.36–1.30) | 0.52 (0.23–1.14) | Ref | 0.71 (0.37–1.38) | 0.52 (0.23–1.16) | Ref | 0.51 (0.22–1.18) | 0.50 (0.21–1.19) | Ref | 0.52 (0.22–1.21) | 0.47 (0.19–1.14) |
| High blood glucose e | Ref | 0.72 (0.33–1.57) | 0.51 (0.21–1.25) | Ref | 0.73 (0.33–1.61) | 0.54 (0.22–1.32) | Ref | 0.91 (0.39–2.10) | 0.69 (0.29–1.65) | Ref | 0.92 (0.39–2.14) | 0.68 (0.28–1.64) |
| Low HDL cholesterolf | Ref | 1.04 (0.48–2.23) | 0.56 (0.21–1.53) | Ref | 1.01 (0.46–2.22) | 0.56 (0.20–1.56) | Ref | 0.32 (0.10–1.05) | 0.39 (0.11–1.35) | Ref | 0.26 (0.07–0.92)* | 0.39 (0.11–1.45) |
| Obesity g | Ref | 0.79 (0.40–1.53) | 0.32 (0.13–0.79)* | Ref | 0.79 (0.40–1.56) | 0.32 (0.13–0.80)* | Ref | 0.58 (0.25–1.36) | 0.40 (0.16–1.00) | Ref | 0.56 (0.24–1.31) | 0.37 (0.15–0.94) |
Notes: Model 1: unadjusted; Model 2: Model 1 + age, smoking, and alcohol.
a ≥3 of 5 components; b ≥102 cm in American men and ≥90 cm in Japanese men; c Systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on medication; d Triglycerides ≥150 mg/dL or on medication; e HDL-cholesterol <40 mg/dL or on medication; f Fasting glucose ≥100 mg/dL or on medication; g BMI ≥30 kg/m2 in American men and BMI ≥25 kg/m2 in Japanese men.
* p < 0.05.
Abbreviations: BMI = body mass index; HDL = high density lipoprotein; LDL = low density lipoprotein; Ref = reference group.
The relationships between individual components of PA from the MAQ (leisure and occupational) and MetS were also examined. There were no associations between self-reported PA levels and MetS in the Japanese men. However, in the American men higher leisure PA (in MET-h/week) was associated with lower odds of having MetS (OR = 0.98, 95%CI: 0.97–0.99; data not shown) and high WC (OR = 0.98, 95%CI: 0.97–0.99; data not shown). There were also no associations between total PA (leisure and occupational) from the MAQ and MetS or individual components of MetS.
4. Discussion
In this comparative study of PA and MetS in a cohort both of U.S. and Japanese men, higher levels of average pedometer steps/day were significantly and strongly predictive of lower odds of having MetS (10%–13% for each 1000 steps/day) and lower odds of having high WC (∼12% for each 1000 steps/day) in both cohorts, as well as lower odds of having high triglycerides in the Japanese cohort (∼7% for each 1000 steps/day). Conversely, an examination of the relationship between domain-specific PA and MetS, using a version of the MAQ specifically modified for applicability to both populations, suggested that leisure PA was only related to lower odds of MetS and lower odds of high WC in the U.S. cohort (both ∼2% for each 1 MET-h/week or 20 min/week of moderate-intensity PA), but not the Japanese cohort. Further examination of the MAQ data to include both leisure and occupational PA did not show a contribution of occupational PA as measured by the MAQ. This finding suggests that when cross-instrument-measured bias in PA reporting is eliminated from the results, total ambulatory movement in both cohorts was more related to MetS and individual MetS components when compared to self-reported domain-specific PA.
A number of previous studies have shown an association between higher step counts and lower odds of MetS in American or Japanese populations.34, 35, 36, 37, 38 However, due to differences in study populations and measurement methods for PA, it is difficult to know whether these findings were “real” or whether they were an artifact caused by differences in how PA data were collected and reported. For example, inclusion criteria and wear time criteria varied by study (e.g., measurement of step counts ranged from only 1 day to 3 consecutive days to 7 consecutive days).
When PA was examined as average total pedometer step counts per day, our study showed that the associations between PA and MetS are very similar for middle-aged men in both countries, using the exact same methods. The study inclusion criteria were also the same in both countries, resulting in a narrow age range of participants, thus reducing confounding by age. Furthermore, compared to previous studies that only used larger cut point categories for reporting step counts, our study showed that even increments of 1000 steps/day were associated with important reductions in the odds of having MetS in both countries, which suggests that step count-based PA intervention efforts to decrease MetS could be impactful in both the United States and Japan.
Our findings suggest that average total step counts/day may be important to the prevalence of MetS in both Japanese and American men. Pedometers are able to capture activities that are difficult to capture via questionnaire (hard to recall), such as unplanned activities that may be of shorter duration and/or lower intensity (such as light-intensity activity). In relation to this, previous studies have shown that light PA,39 and even short bouts of PA such as total PA lasting less than 3 min and moderate to vigorous PA lasting less than 32 s40 are associated with lower prevalence of MetS or individual MetS components. Accumulation of light PA and short bouts of PA that could be captured with pedometer steps but not with the MAQ may have resulted in favorable hormonal profiles in our study population. Because questionnaires are not as good at capturing total ambulatory movement (including unplanned and low-intensity PA),10,13 these findings may help to explain why previously published studies largely based on questionnaire data have shown inconsistent findings across countries for the relationship between PA and MetS.41, 42, 43, 44
Our results suggested that higher levels of leisure PA, collected via the MAQ, were associated with lower odds of MetS in the cohort of American men but not Japanese men. This finding, which showed a smaller effect size than that of total pedometer steps per day, suggests that leisure PA likely contributes to some, but not all, of the effect of total movement (from all intensity levels and domains) on MetS and WC in the American cohort but does not significantly contribute the effect in the Japanese cohort. The fact that there was no significant effect of leisure PA in the Japanese cohort may be due to the much lower reported leisure PA levels among Japanese participants (i.e., leisure PA, as recorded by the MAQ, is not how they accumulated step counts).
This study also illustrates the important differences in patterns of PA between the American and Japanese cohorts, which may explain the inconsistent findings in previous studies regarding the relationship between PA levels and MetS. Depending on the methods of PA assessment, comparison of PA levels have resulted in substantially different findings.5, 6, 7, 8 One study suggested that PA was similar between 2 countries when combined self-reported work and leisure PA was measured.15 However, other studies have suggested that Americans have higher self-reported moderate to vigorous leisure PA8 and lower self-reported occupational PA17 compared to the Japanese. Our study, which confirmed these differences in a standardized setting, highlights the importance of assessing various aspects of PA in cross-country comparisons when the sources of PA differ across populations. Furthermore, our findings suggest that leisure PA levels were more important toward MetS prevalence in the U.S. cohort compared to the Japanese cohort, where the effect was not significant. In our study, the use of the MAQ, which was originally designed to be modified for use across cultures, was effective at capturing these differences but likely did not capture all components of PA important to MetS prevalence in both cohorts. This suggests the need for methods that capture aspects of PA, including light-intensity PA and unplanned activities, that are difficult to assess with questionnaires. However, it should be noted that questionnaires may be useful as a complementary tool to identify the specific components of PA that contribute to the relationship between overall PA levels and MetS.
Our study has several limitations. First, as with most previous studies, the cross-sectional design does not allow us to determine if the relationship between PA and MetS in causal.45 Second, although we used representative samples of populations, our population was limited to middle-aged men at participating sites. Therefore, it is unknown if the results are generalizable to wider Japanese and American populations, which include men in other cities, younger adults, and women. Third, although we adjusted for alcohol use, we did not adjust for other dietary factors. It is possible that some of the association between PA and MetS may be mediated by dietary factors. Finally, there is no gold standard measure for PA that will capture complete information on PA that includes a valid measure of total ambulatory movement and distinguishes the types of activities performed. Therefore, it was necessary to administer both an objective and subjective measure of PA.
However, there are also various strengths to our study. Ours is the first study to compare PA levels in the United States and Japan using both subjective and objective measurement tools. Our study is also the first to assess the association of PA and MetS in the 2 countries simultaneously using standardized methods for outcome collection and processing of PA data. The use of both subjective and objective measures to assess PA enabled us to investigate the associations between MetS and more components of PA. Unlike some studies that measured only 1 day or a few days of pedometer steps, our study used validated methods that only included participants with pedometer steps data for 3 or more of the 7 consecutive days. Also, compared to the age range in previous studies, the age range of our participants was small (13 years), which resulted in less confounding by age.
5. Conclusion
These results showed that average total step counts/day had a strong protective effect on MetS in both the U.S. and Japanese cohorts of middle-aged men, despite differences in other risk factors across the populations. The effect was stronger than any specific component of PA or the combined “total” PA reported through the questionnaire. This suggests that components of PA, such as light-intensity PA and unplanned PA, which can be captured by a pedometer but are difficult to accurately capture with questionnaires, are important in reducing MetS development in both cohorts. Studies that seek to examine the importance of PA levels to MetS prevalence and incidence within and across countries may want to consider using an objective measurement method that can capture total ambulation or total movement across all domains of activity as a way to ensure that all relevant domains of PA are captured. Questionnaires may additionally be valuable to illuminate the contribution of specific components of PA that are important to MetS prevention in each cohort.
Acknowledgments
Acknowledgments
The authors thank the study participants and staff for their contributions. This study was funded by R01 HL68200 from the U.S. National Institutes of Health and by B 16790335 and A 13307016, 17209023, and 21249043 from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Authors’ contributions
NS conceived the study, analyzed and interpreted data, and drafted the manuscript; BRW conceived the study, contributed to data interpretation, and revised the manuscript critically; KA, HU, TH, TT, AES, and KM carried out the study and performed data collection/sorting; AK conceived the study and revised the manuscript critically; AS conceived the study, carried out the study, contributed to interpretation data, and revised the manuscript critically. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.Kassi E., Pervanidou P., Kaltsas G., Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko K.J., Kim E.H., Baek U.H., Gang Z., Kang S.J. The relationship between physical activity levels and metabolic syndrome in male white-collar workers. J Phys Ther Sci. 2016;28:3041–3046. doi: 10.1589/jpts.28.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astrup A., Dyerberg J., Selleck M., Stender S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev. 2008;9(Suppl. 1):S48–S52. doi: 10.1111/j.1467-789X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 4.Lakka T.A., Laaksonen D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 5.Churilla J.R., Fitzhugh E.C. Total physical activity volume, physical activity intensity, and metabolic syndrome: 1999-2004 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2012;10:70–76. doi: 10.1089/met.2011.0057. [DOI] [PubMed] [Google Scholar]
- 6.Kuwahara K., Honda T., Nakagawa T., Yamamoto S., Akter S., Hayashi T. Leisure-time exercise, physical activity during work and commuting, and risk of metabolic syndrome. Endocrine. 2016;53:710–721. doi: 10.1007/s12020-016-0911-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee J., Kim Y., Jeon J.Y. Association between physical activity and the prevalence of metabolic syndrome: from the Korean National Health and Nutrition Examination Survey, 1999-2012. SpringerPlus. 2016;5:1870. doi: 10.1186/s40064-016-3514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen C.B., Nielsen A.J., Bauman A., Tolstrup J.S. Joint association of physical activity in leisure and total sitting time with metabolic syndrome amongst 15,235 Danish adults: a cross-sectional study. Prev Med. 2014;69:5–7. doi: 10.1016/j.ypmed.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Terwee C.B., Mokkink L.B., van Poppel M.N., Chinapaw M.J., van Mechelen W., de Vet H.C. Qualitative attributes and measurement properties of physical activity questionnaires: a checklist. Sports Med. 2010;40:525–537. doi: 10.2165/11531370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Haskell W.L. Physical activity by self-report: a brief history and future issues. J Phys Act Health. 2012;9(Suppl. 1):S5–10. doi: 10.1123/jpah.9.s1.s5. [DOI] [PubMed] [Google Scholar]
- 11.Pereira M.A., Kriska A.M., Collins V.R., Dowse G.K., Tuomilehto J., Alberti K.G. Occupational status and cardiovascular disease risk factors in the rapidly developing, high-risk population of Mauritius. Am J Epidemiol. 1998;148:148–159. doi: 10.1093/oxfordjournals.aje.a009618. [DOI] [PubMed] [Google Scholar]
- 12.Curry W.B., Thompson J.L. Comparability of accelerometer- and IPAQ-derived physical activity and sedentary time in South Asian women: a cross-sectional study. Eur J Sport Sci. 2015;15:655–662. doi: 10.1080/17461391.2014.957728. [DOI] [PubMed] [Google Scholar]
- 13.Strath S.J., Kaminsky L.A., Ainsworth B.E., Ekelund U., Freedson P.S., Gary R.A. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M.D., Cleland V.J., Thomson R.J., Dwyer T., Venn A.J. A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann Epidemiol. 2008;18:378–386. doi: 10.1016/j.annepidem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Stamler J., Elliott P., Dennis B., Dyer A.R., Kesteloot H., Liu K., INTERMAP Research Group INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary) J Hum Hypertens. 2003;17:591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallal P.C., Andersen L.B., Bull F.C., Guthold R., Haskell W., Ekelund U., Lancet Physical Activity Series Working Group Global physical activity levels: surveillance progress, pitfalls, and prospects. The Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 17.Kromhout D., Bloemberg B., Seidell J.C., Nissinen A., Menotti A. Physical activity and dietary fiber determine population body fat levels: the Seven Countries Study. Int J Obes Relat Metab Disord. 2001;25:301–306. doi: 10.1038/sj.ijo.0801568. [DOI] [PubMed] [Google Scholar]
- 18.Bauman A., Bull F., Chey T., Craig C.L., Ainsworth B.E., Sallis J.F. The International Prevalence Study on Physical Activity: results from 20 countries. Int J Behav Nutr Phys Act. 2009;6:21. doi: 10.1186/1479-5868-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 20.Hoang K.C., Le T.V., Wong N.D. The metabolic syndrome in East Asians. J Cardiometab Syndr. 2007;2:276–282. doi: 10.1111/j.1559-4564.2007.07491.x. [DOI] [PubMed] [Google Scholar]
- 21.Sekikawa A., Ueshima H., Kadowaki T., El-Saed A., Okamura T., Takamiya T. Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. Am J Epidemiol. 2007;165:617–624. doi: 10.1093/aje/kwk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekikawa A., Curb J.D., Ueshima H., El-Saed A., Kadowaki T., Abbott R.D. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriska A.M., Knowler W.C., LaPorte R.E., Drash A.L., Wing R.R., Blair S.N. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 24.Schulz L.O., Harper I.T., Smith C.J., Kriska A.M., Ravussin E. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;2:541–548. doi: 10.1002/j.1550-8528.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 25.Kriska A. Ethnic and cultural issues in assessing physical activity. Res Q Exerc Sport. 2000;71(Suppl. 2):S47–S53. [PubMed] [Google Scholar]
- 26.Dunstan D.W., Salmon J., Owen N., Armstrong T., Zimmet P.Z., Welborn T.A. AusDiab Steering Committee. Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia. 2005;48:2254–2261. doi: 10.1007/s00125-005-1963-4. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth B.E., Haskell W.L., Herrmann S.D., Meckes N., Bassett Jr D.R., Tudor-Locke C. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 28.Bassett D.R., Jr, Ainsworth B.E., Leggett S.R., Mathien C.A., Main J.A., Hunter D.C. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28:1071–1077. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Schneider P.L., Crouter S.E., Lukajic O., Bassett D.R., Jr Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc. 2003;35:1779–1784. doi: 10.1249/01.MSS.0000089342.96098.C4. [DOI] [PubMed] [Google Scholar]
- 30.Tudor-Locke C., Burkett L., Reis J.P., Ainsworth B.E., Macera C.A., Wilson D.K. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40:293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Tudor-Locke C., Bassett D.R., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Wen C.P., David Cheng T.Y., Tsai S.P., Chan H.T., Hsu H.L., Hsu C.C. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2009;12:497–506. doi: 10.1017/S1368980008002802. [DOI] [PubMed] [Google Scholar]
- 34.Newton R.L., Jr, Han H., Johnson W.D., Hickson D.A., Church T.S., Taylor H.A. Steps/day and metabolic syndrome in African American adults: the Jackson Heart Study. Prev Med. 2013;57:855–859. doi: 10.1016/j.ypmed.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strath S., Swartz A., Parker S., Miller N., Cieslik L. Walking and metabolic syndrome in older adults. J Phys Act Health. 2007;4:397–410. [PMC free article] [PubMed] [Google Scholar]
- 36.Park S., Park H., Togo F., Watanabe E., Yasunaga A., Yoshiuchi K. Year-long physical activity and metabolic syndrome in older Japanese adults: cross-sectional data from the Nakanojo Study. J Gerontol A Biol Sci Med Sci. 2008;63:1119–1123. doi: 10.1093/gerona/63.10.1119. [DOI] [PubMed] [Google Scholar]
- 37.Sisson S.B., Camhi S.M., Church T.S., Tudor-Locke C., Johnson W.D., Katzmarzyk P.T. Accelerometer-determined steps/day and metabolic syndrome. Am J Prev Med. 2010;38:575–582. doi: 10.1016/j.amepre.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Inoue S., Ohya Y., Tudor-Locke C., Yoshiike N., Shimomitsu T. Step-defined physical activity and cardiovascular risk among middle-aged Japanese: the National Health and Nutrition Survey of Japan 2006. J Phys Act Health. 2012;9:1117–1124. doi: 10.1123/jpah.9.8.1117. [DOI] [PubMed] [Google Scholar]
- 39.Bertrais S., Beyeme-Ondoua J.P., Czernichow S., Galan P., Hercberg S., Oppert J.M. Sedentary behaviors, physical activity, and metabolic syndrome in middle-aged French subjects. Obes Res. 2005;13:936–944. doi: 10.1038/oby.2005.108. [DOI] [PubMed] [Google Scholar]
- 40.Ford E.S., Kohl 3rd H.W., Mokdad A.H., Ajani U.A. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res. 2005;13:608–614. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Young T.K., Zinman B., Harris S.B., Connelly P.W., Hanley A.J. Lifestyle variables, non-traditional cardiovascular risk factors, and the metabolic syndrome in an Aboriginal Canadian population. Obesity (Silver Spring) 2006;14:500–508. doi: 10.1038/oby.2006.65. [DOI] [PubMed] [Google Scholar]
- 42.Fam B., Amouzegar A., Arzhan S., Ghanbariyan A., Delshad M., Hosseinpanah F. Association between physical activity and metabolic risk factors in adolescents: Tehran Lipid and Glucose Study. Int J Prev Med. 2013;4:1011–1017. [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J., Tanabe K., Yokoyama N., Zempo H., Kuno S. Objectively measured light-intensity lifestyle activity and sedentary time are independently associated with metabolic syndrome: a cross-sectional study of Japanese adults. Int J Behav Nutr Phys Act. 2013;10:30. doi: 10.1186/1479-5868-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayabe M., Kumahara H., Morimura K., Ishii K., Sakane N., Tanaka H. Very short bouts of non-exercise physical activity associated with metabolic syndrome under free-living conditions in Japanese female adults. Eur J Appl Physiol. 2012;112:3525–3532. doi: 10.1007/s00421-012-2342-8. [DOI] [PubMed] [Google Scholar]
- 45.Reichenheim M.E., Coutinho E.S. Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression. BMC Med Res Methodol. 2010;10:66. doi: 10.1186/1471-2288-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


