Abstract
Objectives
Serial position effects have been found to discriminate between normal and pathological aging, and to predict conversion from Mild Cognitive Impairment (MCI) to Alzheimer’s disease (AD). Different scoring methods have been used to estimate the accuracy of these predictions. In the current study, we investigated delayed primacy as predictor of progression to early MCI over established diagnostic memory methods. We also compared three serial position methods (regional, standard and delayed scores) to determine which measure is the most sensitive in differentiating between individuals who develop early MCI from a baseline of cognitively intact older adults.
Method
Data were analyzed with binary logistic regression and with receiver-operating characteristic (ROC). Baseline serial position scores were collected using the Rey’s Auditory Verbal Learning Test and used to predict conversion to early MCI. The diagnosis of early MCI was obtained through statistical algorithm and consequent consensus conference. One hundred and ninety-one participants were included in the analyses. All participants were aged 60 or above and cognitively intact at baseline.
Results
The binary logistic regression showed that delayed primacy was the only predictor of conversion to early MCI, when compared to total and delayed recall. ROC curves showed that delayed primacy was still the most sensitive predictor of progression to early MCI when compared to other serial position measures.
Conclusions
These findings are consistent with previous studies and support the hypothesis that delayed primacy may be a useful cognitive marker of early detection of neurodegeneration.
Keywords: Serial position effect, Primacy, Recency, MCI, Cognitive decline, Aging
Introduction
Serial position effects occur in free recall when items presented early on a list (primacy) and items presented at the end of a list (recency) are remembered better than items in the middle (Murdock, 1962). This pattern of performance remains relatively stable during the aging process (Healey & Kahana, 2016; Ward & Maylor, 2005; Wright, 1982), although cognitively healthy older adults tend to forget a greater proportion of words from the middle and recency positions as compared to younger adults (Griffin et al., 2017). However, individuals with Alzheimer’s Disease (AD) show reduced primacy recall relative to both healthy younger and older groups (Burkart, Heun, & Benkert, 1998; Moser et al., 2013), and sometimes show a paradoxical increment of recency recall (Bruno et al., 2016; Greenaway et al., 2006).
Poor primacy performance is also consistently reported (but see Bennet, Golob, Parker & Starr, 2006) when comparing individuals with Mild Cognitive Impairment (MCI), which is considered a stepping stone to AD (Petersen et al., 2001), to healthy age-matched controls (Cunha, Guerreiro, Mendonça, Oliveira & Santana, 2012; Howieson et al., 2011; Shankle et al., 2005); and lower primacy recall has been shown to differentiate between AD and MCI (Cunha et al., 2012). All in all, these findings suggest that performance in the primacy region of recall is more informative than performance in other serial positions, when studying the relationship between episodic memory and cognitive functioning in older adults.
Based on the above, and on their own findings showing that poor primacy performance in cognitively intact elderly predicted cognitive decline, Bruno, Reiss, Petkova, Sidtis, and Pomara (2013) have suggested that primacy may also be a useful predictor of risk of conversion to MCI from a cognitively healthy baseline. Indirect evidence supporting this claim comes from studies showing that serial position effects predict conversion from MCI to AD (Egli et al., 2014). However, the way in which serial position is calculated may affect the aforementioned predictions. For instance, Cunha and colleagues (2012) used two distinct measures of serial position to determine which measure is the most sensitive to neurodegeneration: regional score and standard score. These scores were calculated by dividing the percentage of items recalled from each region by the sum of items presented over the three learning trials in that region (regional) or from the entire list (standard). In the longitudinal study, Cunha and colleagues (2012) investigated the capacity of initial primacy and recency scores to predict conversion to AD, in a group of 134 MCI patients. They first compared serial position effects between MCI-progressives and MCI-nonprogressives and found that both standard and regional recency scores significantly differentiated between the two groups. For comparison, when looking at primacy exclusively, only regional scores were able to statistically differentiate between those who progressed to dementia and those who did not. Subsequently, a receiver operating characteristics (ROC) curve analysis was performed to measure the accuracy of the scoring systems in distinguishing between MCI-progressives and MCI-nonprogressives. The ROC curves showed that primacy was more sensitive than recency in predicting progression to AD when using regional scores, but recency was more sensitive than primacy with standard scores. However, as regional scores were overall more sensitive than standard scores in discriminating progression to AD, Chuna and colleagues (2012), concluded that primacy had potential clinical uses for early detection of AD.
An important consideration regards whether delaying recall increases the accuracy of serial position predictions. Bruno and colleagues (2013) noted that primacy performance after a delay (15–20 min) was a much stronger predictor than any performance measure taken from recall immediately after presentation in cognitively intact individuals, although they did not compare different ways of calculating serial position measures. This finding is consistent with the notion that long-term potentiation and synaptic consolidation require time, and therefore, testing memory after a delay may provide a more truthful assessment of memory ability. Indeed, decreased memory performance at delayed, rather than immediate, recall has been previously found to be predictive of AD (Gomar et al., 2011). Research has found delayed recall to depend mostly on long-term retention and synaptic consolidation, compared to immediate recall (Carlesimo, Marfia, Loasses & Caltagirone, 1996; Dalezman, 1976). As AD pathology originates in the hippocampus (Raj et al., 2015), an area responsible for the storage and consolidation of information, it is plausible that any predictive value of primacy may be enhanced in delayed tasks.
In the current study, we compared delayed primacy to total and delayed recall and to three different serial position scoring methods in a sample of healthy older adults followed for a period of up to 13 years. Our goal was to determine which measure best discriminated between individuals who eventually developed early symptoms of MCI, and those who maintained normal cognitive functioning. For the purposes of the present study individuals with more severe classifications (e.g., dementia) were excluded. However Johnson and colleagues (2018), using the Wisconsin Registry for Alzheimer’s Prevention (WRAP) dataset, have shown that non-clinical individuals presenting early symptoms of MCI at baseline were associated with higher risk of progressing to MCI or dementia at their last visit than compared to individuals who were cognitively intact, therefore suggesting the early MCI designation has predictive value (details on early MCI diagnosis in the Procedure section). We hypothesized that delayed primacy performance would be the strongest measure for predicting those who exhibited early MCI at their last assessment among those who were unimpaired through all visits.
Methods
Participants
Participants were selected from the WRAP, an ongoing longitudinal cohort study examining cognitive trajectories and associated risk factors in a sample of older adults with or without family history of AD. The first follow-up occurs at least after 4 years, while all subsequent visits occur at 2-years intervals thereafter. Participants for this study were selected on the basis of having completed at least two visits, having received a diagnosis of normal cognitive functioning at baseline, and either being classified as still cognitively normal or with possible early MCI at their last visit (details on diagnoses in the Procedure section). Additionally, at baseline, selected participants were free of neurological diseases and psychiatric disorders, spoke English as their native language, and were 60 or older. From the total pool of 1551 volunteers, we analyzed data from 191 participants who fulfilled the above inclusion criteria. Specifically, 46 participants completed five visits, 77 completed four visits, 48 came back for three visits 20 came back for one follow-up. Of the 191 participants, 179 were classified as White/Caucasian, 10 were Black/African American, 1 was Spanish/Hispanic and 1 was Asian. All activities for this study were approved by the ethics committees of the authors’ universities, and completed in accordance with the Helsinki Declaration.
Procedure
Each WRAP participant completed an entry assessment that included laboratory tests, clinical measurements, and a health history and lifestyle form assessing demographics, self-reported medical and psychiatric status and depressive symptoms (20-item Center for Epidemiologic Studies-Depression Scale [CES-D]). WRAP adopts a two-tiered consensus conference method to classify individuals’ cognitive status. The first tier of review includes applying a statistical algorithm that identifies cases where impairment may be present; and the second tier includes a team review of those flagged by the algorithm. Specifically, WRAP participants’ visits are reviewed at a consensus case conference if they meet one or more of the following criteria: (1) the participant is performing 1.5 SDs below the mean on factor scores or individual measures of memory, executive function, language, working memory, or attention (Clark et al., 2016; Koscik et al., 2014); (2) cognitive performance on one or more tests fell below values used in other studies as cut-points for clinical MCI diagnoses (e.g., WMS-R Logical Memory II, Wechsler, 1987: story A score <9: Alzheimer’s Disease Neuroimaging Initiative, Petersen et al., 2010); or (3) an abnormal informant report indicating subjective cognitive or functional decline. Consensus diagnoses are determined for each visit by a research team including physicians, clinical neuropsychologists, and clinical nurse practitioners based on review of cognitive, medical history, lifestyle, subjective cognitive complaints, and informant data (Koscik et al., 2016). The status of early MCI was developed to identify individuals in the cohort whose objective performance is at least 1.5 SDs below expected in one or more cognitive domains relative to internal robust norms, but who do not demonstrate clinical levels of cognitive deficits nor have subjective reports of cognitive complaint, fundamental for MCI diagnosis (Albert et al., 2011). This experimental construct is thought to represent a phenotype of early cognitive decline expected to precede a clinical diagnosis of MCI. This category broadly corresponds to clinical stage 2 in the 2018 diagnostic framework (Jack et al. 2018).
The neuropsychological battery comprised commonly used clinical tests (see Sager, Hermann and La Rue, 2005; Johnson et al., 2018 for a description of the baseline cognitive battery), including measures used as cognitive outcomes for this study: Trail Making Test A and B (Reitan, 1986) and Stroop Color Word Test (Stroop, 1935) for working memory and executive abilities; Wechsler Abbreviated Scale of Intelligence [WASI] Vocabulary and Similarities subtests (Wechsler, 1999), Boston Naming Test (BNT; Kaplan, Goodglass & Weintraub, 2001) and Wide Range Achievement Test [WRAT] Reading Test (Robertson, 2009) for language and verbal skills; WASI Block Design subtest, WASI Matrix Reasoning and Judgment of Line Orientation (JLO; Benton, Hamsher, Varney & Spreen, 1983) for visuospatial abilities; working memory subtests of the Wechsler Adult Intelligence Scale-III (WAIS; Wechsler, 1999) for working memory; and Rey Auditory Verbal Learning Test, (AVLT; Schmidt, 1996) for verbal and visual episodic memory.
In the AVLT, a list of 15 semantically unrelated words is orally presented to the participants in an initial trial, after which participants are required to freely recall the words. Subsequently, four more identical trials are performed, for a total of five learning trials (total recall). After these trials, a distractor list of 15 different and unrelated words is presented and subjects are asked to recall it. Finally, after a delay of approximately 20 min, participants are asked to freely recall items from the first presented list: this is referred to as the delayed recall.
To calculate the serial position scores, we divided the 15-word list as follows: primacy was characterized by the first 4 words of the list, whilst recency was composed of the last 4 (Foldi, Brickman, Schaefer, & Knutelska, 2003; Hermann et al. 1996; La Rue et al. 2008). Due to increasing evidence that primacy and recency, rather than middle-items, may be more influential in detecting cognitive decline, we focused on these two components. Primacy and recency scores were calculated in each learning trial, summed up and then divided by the total possible number of primacy and recency words recalled (20). In line with Chuna and colleagues (2012), primacy and recency regional and standard scores were derived from the five learning trials. Due to the fact that delayed recall consists of only one trial, it was not possible to use the same formulas for delayed primacy and recency. In line with the literature (Hermann et al. 1996; Kasper et al., 2016; La Rue et al. 2008), delayed primacy and recency were defined as the proportion of correctly recalled primacy and recency items from the delayed trial. For instance, participant A recalled 6 words from trial 1, 9 from trial 2, 10 from trial 3, 9 from trial 4, 10 from trial 5, for a total of 44 words recalled. Of the four words that could be recalled from the primacy region at each of the five trials, participant A recalled 2 at trial 1, 2 at trial 2, 4 at trial 3, 3 at trial 4, 4 at trial 5. Regional primacy is calculated as:
Standard primacy is calculated as:
Participant A also recalled 3 (out of 4) words from the primacy region at delayed recall, therefore delayed primacy is calculated as:
Outcome measures were primacy and recency regional and standard scores, as well as delayed primacy and recency. In order to compare the initial level of cognitive functioning between participants who progressed to early MCI and those who did not, a composite score for general cognitive ability variable was calculated as the average of four baseline cognitive factor z-scores: (1) Speed and Flexibility, obtained using the TMT-A and B and the Stroop Color-Word test; (2) Verbal Abilities, obtained with the WASI vocabulary, WASI similarities, BNT and WRAT; (3) Visuospatial Abilities, using the WASI block design, matrix reasoning and the JLO; and (4) Working Memory, obtained using the digit span back and forward and the letter-number sequencing (details on the factor analyses can be found in Dowling, Hermann, La Rue & Sager, 2010 and Koscik et al., 2014). Generally, the consensus diagnosis for early MCI included evaluation of two measures from AVLT test (excluding serial position data), as part of the 15–20 of the cognitive measures considered (e.g., executive function impairments or language impairments were also evaluated). We feel, therefore, that even though predictors and outcome cannot be thought of as being 100% “conceptually” independent, the level of circularity is relatively low.
Statistical Analysis
All the analyses were performed using SPSS, Version 23 (IBM). Variables included in the main analysis were total and delayed recall and primacy and recency, calculated in standard, regional and delayed scores. As the serial position scores were not normally distributed, we utilized non-parametric analyses when these variables were included. Parametric analyses were used for normally distributed data.
First, each measurement of primacy and recency was correlated to general cognitive ability in both groups to assess their sensitivity to global cognition. Subsequently, we ran Mann–Whitney tests and t-test to determine if there were differences in demographics, cognitive level and serial position performance between those who progressed to early MCI and those who did not. Therefore, we tested our hypothesis that delayed primacy is a stronger measure in predicting progression to early MCI compared to clinically used measures of cognitive decline through a binary logistic regression with delayed primacy as predictor, and progression to early MCI or not as binary outcome, whilst controlling for immediate and delayed total recall, respectively. Since time between first and last visit was not the same for each participant, time was also added as control variable, together with age, gender, APOE4 status, and years of education. To avoid issues of multicollinearity, delayed primacy was regressed out of AVLT delayed recall, yielding standardized residuals; in turn, delayed primacy and the AVLT delayed recall standardized residuals were regressed out of AVLT total recall, generating also standardized residuals for AVLT total recall. A Spearman’s correlation analysis confirmed the independence of these measures.
Finally, we followed Chuna and colleagues (2012) statistical method and generated receiver-operating characteristic (ROC) curves to check for specificity and sensitivity of delayed primacy, compared to regional and standard serial position calculations.
Results
Table 1 summarizes the subjects’ demographic characteristics and comparisons for cognitive and memory scores.
Table 1.
Demographics, cognitive level, and serial position performance between MCI-progressive and MCI-nonprogressive participants
| Early MCI-p (n = 33) | Controls (n = 158) | ρ value | |
|---|---|---|---|
| Gender (females) | 25 (76%) | 111 (70%) | .526 |
| APOE4 presence | 7 (22%) | 44 (28%) | .434 |
| Age | 62.58 ± 1.80 | 62.23 ± 1.95 | .346 |
| Years of education | 16.59 ± 2.99 | 16.34 ± 3.00 | .665 |
| General cognitive abilities | −0.40 ± 0.53 | −0.11 ± 0.60 | .001 |
| Time | 7.97 ± 2.56 | 7.94 ± 2.10 | .952 |
| AVLT Total Recall | 47 (35–60) | 51.50 (35–68) | .001 |
| AVLT Delayed Recall | 10 (5–15) | 11 (6–15) | .016 |
| Serial position effect measures: | |||
| Primacy | |||
| Regional scores | 0.65(1–0.20) | 0.70(1–0.40) | .227 |
| Standard scores | 0.29 (0.49–0.20) | 0.29 (0.42–0.14) | .690 |
| Delayed scores | 0.75 (1–0) | 0.75 (1–0.25) | .003 |
| Recency | |||
| Regional scores | 0.15 (0.20–0) | 0.15 (0.20–0) | .186 |
| Standard scores | 0.33 (0.40–0.18) | 0.32 (0.51–0.19) | .953 |
| Delayed scores | 0.50 (0–0) | 0.50 (0–0) | .570 |
Note: Early MCI-p = mild cognitive impairment progressive; Controls = non progressive to early MCI; Time = time between first visit and last follow-up.
One hundred fifty eight (82.7%) of the 191 participants remained cognitively intact at the last visit, whereas 33 (17.3%) progressed to Early MCI. Of these, 14 met psychometric amnestic MCI criteria and nine met non-amnestic MCI criteria (Koscik et al., 2014) at their last visit. 71% of the total sample was female, mean age at baseline was 62.29 (SD = 1.92), with 16.38 (SD = 2.99) total years of education. Correlation analyses between general cognitive abilities and serial position measures are shown in Table 2. General cognitive abilities were positively related to delayed primacy, ρ(184) = .218, p = .003, and recency scores, ρ(184) = .196, p = .008, and with primacy regional scores, ρ(184) = .235, p = .001.
Table 2.
Spearman correlation between cognitive performance and serial position measures
| Measure | Cognitive ability |
|---|---|
| Regional primacy | .235* |
| Regional recency | .054 |
| Standard primacy | .051 |
| Standard recency | −.123 |
| Delayed primacy | .218* |
| Delayed recency | .196* |
Note: Regional scores = primacy/recency words recalled divided by total number of primacy/recency words presented; Standard scores = primacy/recency words recalled divided by total number of words recalled; Delayed scores = proportion of delayed words recalled for primacy and recency; * = correlation significant at p < .01.
As cognitive ability was normally distributed, an independent samples t-test was performed to compare the initial cognitive functioning of the two groups and results showed a statistically significant difference between MCI progressive (M = –0.404, SD = .53) and MCI-nonprogressives (M = –0.11, SD = .60), t (184) = 3.471, p = .001. As data were not normally distributed, Mann–Whitney tests were run to determine if there were differences between the two groups in memory measures, including immediate and delayed total recall and serial position effects measures. Statistically significant differences on memory measures were found for delayed primacy (U = 1820.500, z = –2.933, p = .003) between MCI-progressive (median = .75, range = .25–1.00, mean rank = 72.17) and MCI-nonprogressive (median = .75, range = .25–1.00, mean rank = 100.98) participants. Significant differences were also found for immediate total recall (U = 1617.500, z = –3.429, p = .001) between MCI-progressive (median = 47, range = 35–60, mean rank = 66.02) and MCI-nonprogressive (median = 51.50, range = 35–68, mean rank = 102.26) individuals. Lastly, delayed total recall was statistically lower in the MCI-progressive (median = 10, range = 5–15, mean rank = 75.08) compared to the MCI-nonprogressive (median = 11, range = 6–15, mean rank = 100.37) group (U = 1916.500, z = –2.408, p = .02). Spearman’s correlation was performed in order to guarantee that there was no correlation between delayed recall and total recall (rs(189) = –.004, p = .953), between delayed recall and delayed primacy (rs(189) = .074, p = .308), and between total recall and delayed primacy (rs(189) = .010, p = .896). The logistic regression model was statistically significant, χ2(8) = 16.951, p = .031. The model explained 14% (Nagelkerke R2) of the variance in conversion to early MCI and correctly classified 84% of cases. Delayed primacy was the only statistically significant measure to predict progression to early MCI, compared to the other control variables, as shown in Table 3.
Table 3.
Logistic regression predicting progression to early MCI based on delayed primacy performance at baseline
| Measures | B | SE | Wald | p | Exp(B) |
|---|---|---|---|---|---|
| Delayed primacy | −3.452 | 1.044 | 10.925 | .001 | 0.496 |
| AVLT Total Recall | −0.093 | .214 | 0.191 | .662 | 0.911 |
| AVLT Delayed Recall | −0.314 | 0.224 | 1.977 | .160 | 0.730 |
| Time | 0.003 | 0.002 | 3.170 | .075 | 1.003 |
| Age | 0.194 | 0.204 | 0.910 | .340 | 1.106 |
| Years of education | 0.250 | 0.212 | 1.393 | .238 | 1.086 |
| Sex | 0.925 | 0.525 | 3.107 | .078 | 2.522 |
| APOE4 | −0.479 | 0.508 | 0.887 | .346 | 0.620 |
Note. B = unstandardized regression coefficient; SE = standard error of the coefficient; Exp(B) = odds ratio; Time = time between first visit and last follow-up; AVLT Total and Delayed Recall = standardized residuals.
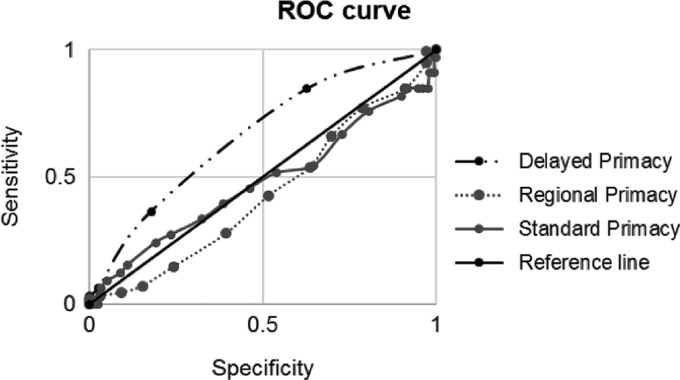
The ROC curves (Table 4) showed that, compared to the other serial position measures, delayed primacy was the only statistically significant measure able to discriminate between those who converted to early MCI, with 65% accuracy. Fig. 1 shows areas under the ROC curves for delayed primacy, compared to regional and standard primacy.
Table 4.
Areas under the ROC curve by serial position scoring measures in progressive MCI and controls
| Early MCI-p vs. Controls | |||
|---|---|---|---|
| Measures | AUC | p | 95% CI |
| Regional primacy | .57 | .230 | [.45, .68] |
| Regional recency | .57 | .215 | [.46, .67] |
| Standard primacy | .48 | .691 | [.36, .60] |
| Standard recency | .50 | .953 | [.39, .61] |
| Delayed primacy | .65 | .006 | [.55, .75] |
| Delayed recency | .53 | .587 | [.43, .63] |
Note: Early MCI-p = progressed to early mild cognitive impairment; controls = non progressive MCI. ROC = receiver-operating characteristic. AUC = area under the curve. CI = confidence interval.
Fig. 1.
Receiver Operating Characteristic (ROC) curve of delayed, regional and standard primacy measures.
Diagnostic concordance was also assessed using positive predictive values (PPV) and negative predictive values (NPV). With a cut-off of 1 out of 4 on delayed primacy, the PPV was 33% and the NPV was 83% (sensitivity 6%, specificity 97%), whilst a cut-off of 2 out of 4, produced a PPV of 30% and a NPV of 86% (sensitivity 36%, specificity 82%).
Discussion
The current study was the first, to the best of our knowledge, to compare several memory and serial position measures and their sensitivity in predicting progression to early MCI from a baseline of intact cognition. In our results, as per our hypothesis, delayed primacy is the strongest memory measure in predicting conversion to early MCI from a cognitively intact baseline, when compared to established diagnostic memory methods, such as AVLT total and delayed recall. The ROC analysis showed that delayed primacy is still the only significant measure also when compared to other serial position calculations, although falling in the “poor” range. The general findings are consistent with Bruno and colleagues (2013) results, showing in a longitudinal study that delayed primacy recall was the most sensitive predictor of cognitive decline in cognitively intact older adults, when compared to total recall, middle and recency, and when evaluating both immediate memory (i.e., learning trials) and delayed performance.
None of the control variables were found to predict conversion to early MCI. This may be due to our sample being heterogeneous for age, years of education and sex. Moreover, APOE4 has been found to have limited value for predicting dementia in clinical practice (for a review, see: Elias-Sonnenschein, Viechtbauer, Ramakers, Verhey & Visser, 2011), therefore it is not surprising that significance was not reached in our model.
Lower delayed primacy performance is associated with an increased likelihood of conversion to early MCI. The low PPV values indicate that the conversion is not imperative (33% or 30%), however the relatively high rate of false positives shows that there is higher chance (83% or 86%) that the person with higher delayed primacy may not progress to early MCI. These findings also align with the sensitivity and specificity of the cut-off reported for delayed primacy. Therefore, high delayed primacy may play a protective factor for cognitive decline, thus suggesting potential implications for neuropsychological assessments. For instance, delayed primacy may be considered among other cognitive markers for the purposes of determining who is suitable (i.e., at greater risk of conversion) for inclusion in clinical trials for AD drugs.
Primacy effects have been characteristically linked to increased opportunities for rehearsal relative to other portions of the serial position curve (Rundus, 1971; Tan & Ward, 2000), although primacy effects have also been observed without rehearsal (Wright, 1994). Indeed, Bruno and colleagues (2015) have previously suggested that a mechanism involving opportunities to rehearse and later consolidate the information, relying on a pathway involving the prefrontal cortex and the hippocampus, may explain the predictive strength of delayed primacy recall – as we have also argued in the Introduction. However, Bruno and colleagues (2016) argued that delayed primacy recall could be parsed into two separate mechanisms. First, a tendency to begin recall by retrieving items from the beginning of the list – tendency that is emphasized in delayed trials, where short-term, recency-based effects dissipate. Second, the ability to use temporal context from retrieved items to cue the recall of temporally contiguous stimuli. While the latter appears to rely on hippocampal function, the former is more uniquely associated to primacy. Alternatively, it is also possible that primacy is a valuable predictor of cognitive decline, specifically at delayed recall, because it taps into the individual’s ability to focus attention during learning (Sederberg et al., 2006). In this regard, it is to note also how APOE4 carries show attentional deficits in working memory tasks (Rosen, Bergeson, Putham, Harwell & Sunderland, 2002). Similarly, primacy may also be involved in processing novelty items (Davelaar, 2013) and/or changes in contextual information (Howard & Kahana, 2002). More research is needed to address these questions.
Our ROC analysis findings on standard and regional scores yielded lower diagnostic power compared to previous reports on clinical population (Cunha et al., 2012; Egli et al., 2014), where serial position measures were used to investigate conversion from MCI to AD. Specifically, Cuhna et al.’s ROC analysis showed an accuracy falling in the “fair” range for both regional primacy (70%) and standard recency (68%), compared to the “poor” range of delayed primacy (65%). The lower accuracy may be due to the fact that our sample was composed of healthy participants who converted to a pre-clinical stage, whilst previous studies (Cunha et al., 2012; Egli et al., 2014) investigated an already clinical population who progressed to dementia. Furthermore, the size of our sample was not equal across groups, with a larger proportion of controls compared to those who progressed to early MCI, which may reduce the comparability to studies with different samples. For instance, Cunha and colleagues (2012) reported that the number of individuals with MCI who progressed to dementia was higher than the number of those who did not. This discrepancy is not surprising since it is more likely that individuals with MCI progress to any type of dementia, rather than healthy individuals develop MCI. Indeed, other studies investigating progression to early MCI reported similar conversion rates (14% in Bruno, Koscik, Woodard, Pomara & Johnson, 2018; 15.2% in Johnson et al., 2018). Moreover, the years of education of individuals with AD and MCI in Cunha and colleagues (2012) was relatively low, (M = 5.20, SD = 3.97 and M = 6.78, SD = 4.32) compared to our sample (M = 16.59, SD = 2.99). Since education is an important protective factor for cognitive decline, the higher education of our participants may have contributed to the lower conversion to early MCI. Although Egli and colleagues (2015) and Howieson and colleagues (2011) considered delayed recall performance in comparing serial position measures, they did not specifically isolate either delayed primacy or delayed recency.
There are some limitations in the present study that may be taken into account for future directions. Firstly, the main analyses did not include information regarding sub-categories of MCI due to the fact that these diagnoses are based on performances on the AVLT, which would have brought circularity problems for the statistical design. Future studies may consider to include these classifications if possible. Secondly, not all participants with early MCI progress to clinical stages, therefore caution should be used in generalizing the findings. Moreover, the algorithm used for the consensus conference process does not include any criteria for subjective self-report of memory problems. Therefore, the early MCI group may miss individuals who have subtle decline that is not detected by the flagging algorithm. Lastly, although delayed primacy was the only statistically significant serial position measure in the ROC analysis, it yielded low diagnostic power. However, it should be noted that we examined prediction of conversion to early MCI, which is a pre-clinical classification. Therefore, further studies should consider investigating the appropriateness of delayed primacy, compared to other serial position measures, for prediction of clinical MCI from a healthy baseline.
So far, this is the first study to longitudinally investigate conversion to early MCI from a stage of intact cognitive functioning level and to compare the efficacy of several serial position measures. In line with previous research on healthy and declining population, we found that delayed primacy is the most accurate scoring measure in predicting progression to an early stage of MCI, even when considering memory measures broadly utilized for neuropsychological assessment, such as total and delayed recall. Since MCI is a crucial stage for dementia development, further research may focus on its early stage to improve early detection of cognitive impairment in clinical settings, and to allow early neuropsychological interventions. Being able to predict early MCI from a cognitive functioning stage may help prevent, or delay, its clinical appearance and, consequently, its progression to dementia.
Acknowledgements
We wish to thank Mr Allen Wenzel for helping with data management, the WRAP study team for their data collection efforts and the WRAP participants for their dedication to the study. We also wish to thank Dr Mark Forshaw and Dr Ruth Ogden for providing supervision on the project.
Funding
This work was supported by an Early Career Researcher Studentship Award and by a National Institute on Aging [R01AG27161]. This study was also supported by the Clinical and Translational Science Award program, through the National Institute of Health National Center for Advancing Translational Sciences [UL1TR000427]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
None declared.
References
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett I. J., Golob E. J., Parker E. S., & Starr A. (2006). Memory evaluation in mild cognitive impairment using recall and recognition tests. Journal of Clinical and Experimental Neuropsychology, 28, 1408–1422. [DOI] [PubMed] [Google Scholar]
- Benton A. L., Hamsher K. D., Varney N. R., & Spreen O. (1983). Judgment of line orientation. New York: Oxford University Press. [Google Scholar]
- Bruno D., Grothe M. J., Nierenberg J., Sidtis J. J., Teipel S. J., & Pomara N. (2016. a). Output order and variability in free recall are linked to cognitive ability and hippocampal volume in elderly individuals. Neuropsychologia, 80, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D., Grothe M. J., Nierenberg J., Zetterberg H., Blennow K., Teipel S. J., et al. (2015). A study on the specificity of the association between hippocampal volume and delayed primacy performance in cognitively intact elderly individuals. Neuropsychologia, 69, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D., Koscik R. L., Woodard J. L., Pomara N., & Johnson S. C. (2018). The recency ratio as predictor of early MCI. International Psychogeriatrics, 30(12), 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D., Reichert C., & Pomara N. (2016. b). The recency ratio as an index of cognitive performance and decline in elderly individuals. Journal of Clinical and Experimental Neuropsychology, 38, 967–973. [DOI] [PubMed] [Google Scholar]
- Bruno D., Reiss P. T., Petkova E., Sidtis J. J., & Pomara N. (2013). Decreased recall of primacy words predicts cognitive decline. Archives of Clinical Neuropsychology, 28, 95–103. [DOI] [PubMed] [Google Scholar]
- Burkart M., Heun R., & Benkert O. (1998). Serial position effects in dementia of the Alzheimer type. Dementia and Geriatric Cognitive Disorders, 9, 130–136. [DOI] [PubMed] [Google Scholar]
- Carlesimo G. A., Marfia G. A., Loasses A., & Caltagirone C. (1996). Recency effect in anterograde amnesia: Evidence for distinct memory stores underlying enhanced retrieval of terminal items in immediate and delayed recall paradigms. Neuropsychologia, 34, 177–184. [DOI] [PubMed] [Google Scholar]
- Clark L. R., Koscik R. L., Nicholas C. R., Okonkwo O. C., Engelman C. D., Bratzke L. C., et al. (2016). Mild cognitive impairment in late middle age in the Wisconsin Registry for Alzheimer’s Prevention Study: Prevalence and characteristics using robust and standard neuropsychological normative data. Archives of Clinical Neuropsychology, 31, 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C., Guerreiro M., de Mendonça A., Oliveira P. E., & Santana I. (2012). Serial position effects in Alzheimer’s disease, mild cognitive impairment, and normal aging: Predictive value for conversion to dementia. Journal of Clinical and Experimental Neuropsychology, 34, 841–852. [DOI] [PubMed] [Google Scholar]
- Dalezman J. J. (1976). Effects of output order on immediate, delayed, and final recall performance. Journal of Experimental Psychology: Human Learning and Memory, 2, 597. [Google Scholar]
- Davelaar E. J. (2013). A novelty-induced change in episodic (NICE) context account of primacy effects in free recall. Psychology (Savannah, Ga.), 4, 695. [Google Scholar]
- Dowling N. M., Hermann B., La Rue A., & Sager M. A. (2010). Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology, 24, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli S. C., Beck I. R., Berres M., Foldi N. S., Monsch A. U., & Sollberger M. (2014). Serial position effects are sensitive predictors of conversion from MCI to Alzheimer’s disease dementia. Alzheimer’s & Dementia, 10, S420–S424. [DOI] [PubMed] [Google Scholar]
- Egli S. C., Hirni D. I., Taylor K. I., Berres M., Regeniter A., Gass A., et al. (2015). Varying strength of cognitive markers and biomarkers to predict conversion and cognitive decline in an early-stage-enriched mild cognitive impairment sample. Journal of Alzheimer’s Disease, 44, 625–633. [DOI] [PubMed] [Google Scholar]
- Elias-Sonnenschein L. S., Viechtbauer W., Ramakers I. H., Verhey F. R., & Visser P. J. (2011). Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: A meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry, 82, 1149–1156. jnnp-2010. [DOI] [PubMed] [Google Scholar]
- Foldi N. S., Brickman A. M., Schaefer L. A., & Knutelska M. E. (2003). Distinct serial position profiles and neuropsychological measures differentiate late life depression from normal aging and Alzheimer’s disease. Psychiatry Research, 120, 71–84. [DOI] [PubMed] [Google Scholar]
- Gomar J. J., Bobes-Bascaran M. T., Conejero-Goldberg C., Davies P., Goldberg T. E., & Alzheimer’s Disease Neuroimaging Initiative. (2011). Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Archives of General Psychiatry, 68, 961–969. [DOI] [PubMed] [Google Scholar]
- Greenaway M. C., Lacritz L. H., Binegar D., Weiner M. F., Lipton A., & Cullum C. M. (2006). Patterns of verbal memory performance in mild cognitive impairment, Alzheimer disease, and normal aging. Cognitive and Behavioral Neurology, 19, 79–84. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., John S. E., Adams J. W., Bussell C. A., Saurman J. L., & Gavett B. E. (2017). The effects of age on the learning and forgetting of primacy, middle, and recency components of a multi-trial word list. Journal of Clinical and Experimental Neuropsychology, 39(9), 900–912. [DOI] [PubMed] [Google Scholar]
- Healey M. K., & Kahana M. J. (2016). A four-component model of age-related memory change. Psychological Review, 123, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B. P., Seidenberg M., Wyler A., Davies K., Christeson J., Moran M., et al. (1996). The effects of human hippocampal resection on the serial position curve. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 32, 323–334. [DOI] [PubMed] [Google Scholar]
- Howard M. W., & Kahana M. J. (2002). A distributed representation of temporal context. Journal of Mathematical Psychology, 46, 269–299. [Google Scholar]
- Howieson D. B., Mattek N., Seeyle A. M., Dodge H. H., Wasserman D., Zitzelberger T., et al. (2011). Serial position effects in mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology, 33, 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R. J., Bennett D. A., Blennow K., Carrillo M. C., Dunn B., Haeberlein S. B., et al. (2018). NIA-AA research framework: towards a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia, 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Koscik R. L., Jonaitis E. M., Clark L. R., Mueller K. D., Berman S. E., et al. (2018). The Wisconsin Registry for Alzheimer’s prevention: A review of findings and current directions. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 10, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., & Weintraub S. (2001). Boston naming test. Pro-ed.
- Kasper E., Brueggen K., Grothe M. J., Bruno D., Pomara N., Unterauer E., et al. (2016). Neuronal correlates of serial position performance in amnestic mild cognitive impairment. Neuropsychology, 30, 906. [DOI] [PubMed] [Google Scholar]
- Koscik R. L., Berman S. E., Clark L. R., Mueller K. D., Okonkwo O. C., Gleason C. E., et al. (2016). intraindividual cognitive variability in middle age predicts cognitive impairment 8–10 years later: Results from the Wisconsin Registry for Alzheimer’s prevention. Journal of the International Neuropsychological Society, 22, 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik R. L., La Rue A., Jonaitis E. M., Okonkwo O. C., Johnson S. C., Bendlin B. B., et al. (2014). Emergence of mild cognitive impairment in late middle-aged adults in the wisconsin registry for Alzheimer’s prevention. Dementia and Geriatric Cognitive Disorders, 38, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A., Hermann B., Jones J. E., Johnson S., Asthana S., & Sager M. A. (2008). Effect of parental family history of Alzheimer’s disease on serial position profiles. Alzheimer’s & Dementia, 4, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Deisenhammer E. A., Marksteiner J., Papousek I., Fink A., & Weiss E. M. (2013). Serial position effects in patients with mild cognitive impairment and early and moderate Alzheimer’s disease compared with healthy comparison subjects. Dementia and Geriatric Cognitive Disorders, 37, 19–26. [DOI] [PubMed] [Google Scholar]
- Murdock B. B., Jr. (1962). The serial position effect of free recall. Journal of Experimental Psychology, 64, 482. [Google Scholar]
- Petersen R. C., Aisen P. S., Beckett L. A., Donohue M. C., Gamst A. C., Harvey D. J., et al. (2010). Alzheimer’s disease Neuroimaging Initiative (ADNI) clinical characterization. Neurology, 74, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C., Stevens J. C., Ganguli M., Tangalos E. G., Cummings J. L., & DeKosky S. T. (2001). Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 56, 1133–1142. [DOI] [PubMed] [Google Scholar]
- Raj A., LoCastro E., Kuceyeski A., Tosun D., Relkin N., Weiner M., & Alzheimer’s Disease Neuroimaging Initiative (ADNI). (2015). Network diffusion model of progression predicts longitudinal patterns of atrophy and metabolism in Alzheimer’s disease. Cell Reports, 10, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. M. (1986). Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory.
- Robertson G. J. (2009). Wide‐Range Achievement Test. Corsini Encyclopedia of Psychology.
- Rosen V. M., Bergeson J. L., Putnam K., Harwell A., & Sunderland T. (2002). Working memory and apolipoprotein E: What’s the connection? Neuropsychologia, 40, 2226–2233. [DOI] [PubMed] [Google Scholar]
- Rundus D. (1971). Analysis of rehearsal processes in free recall. Journal of Experimental Psychology, 89, 63. [Google Scholar]
- Sager M. A., Hermann B., & La Rue A. (2005). Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Journal of Geriatric Psychiatry and Neurology, 18, 245–249. [DOI] [PubMed] [Google Scholar]
- Schmidt M. (1996). Rey auditory verbal learning test: A handbook. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sederberg P. B., Gauthier L. V., Terushkin V., Miller J. F., Barnathan J. A., & Kahana M. J. (2006). Oscillatory correlates of the primacy effect in episodic memory. Neuroimage, 32, 1422–1431. [DOI] [PubMed] [Google Scholar]
- Shankle W. R., Romney A. K., Hara J., Fortier D., Dick M. B., Chen J. M., et al. (2005). Methods to improve the detection of mild cognitive impairment. Proceedings of the National Academy of Sciences of the United States of America, 102, 4919–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643. [Google Scholar]
- Tan L., & Ward G. (2000). A recency-based account of the primacy effect in free recall. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 1589. [DOI] [PubMed] [Google Scholar]
- Ward G., & Maylor E. A. (2005). Age-related deficits in free recall: The role of rehearsal. The Quarterly Journal of Experimental Psychology Section A, 58, 98–119. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1987). WMS-R: Wechsler memory scale-revised. Psychological Corporation.
- Wechsler D. (1999). Manual for the Wechsler abbreviated intelligence scale (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wright R. E. (1982). Adult age similarities in free recall output order and strategies. Journal of Gerontology, 37, 76–79. [DOI] [PubMed] [Google Scholar]
- Wright A. A. (1994). Primacy effects in animal memory and human nonverbal memory. Animal Learning & Behavior, 22, 219–223. [Google Scholar]