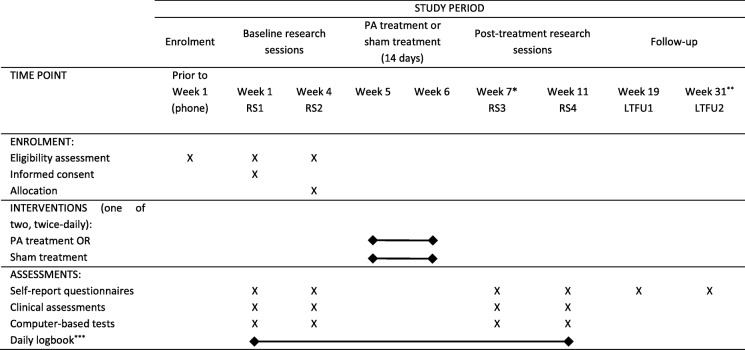
Table 1.
Schedule of enrolment, interventions, and assessments for the participants with CRPS
RS Research Session, LTFU Long Term Follow-Up (postal questionnaires only), PA Prism Adaptations
*Primary endpoint of the study
**Secondary endpoint of the study
***Self-reported average levels of pain, range of movement and symptoms interference with daily life in the last 24 h, rated daily on 0–10 Numeric Rating Scales