Figure 1: Keratinocytes are equipped to sense tissue status and maintain T cell niche.
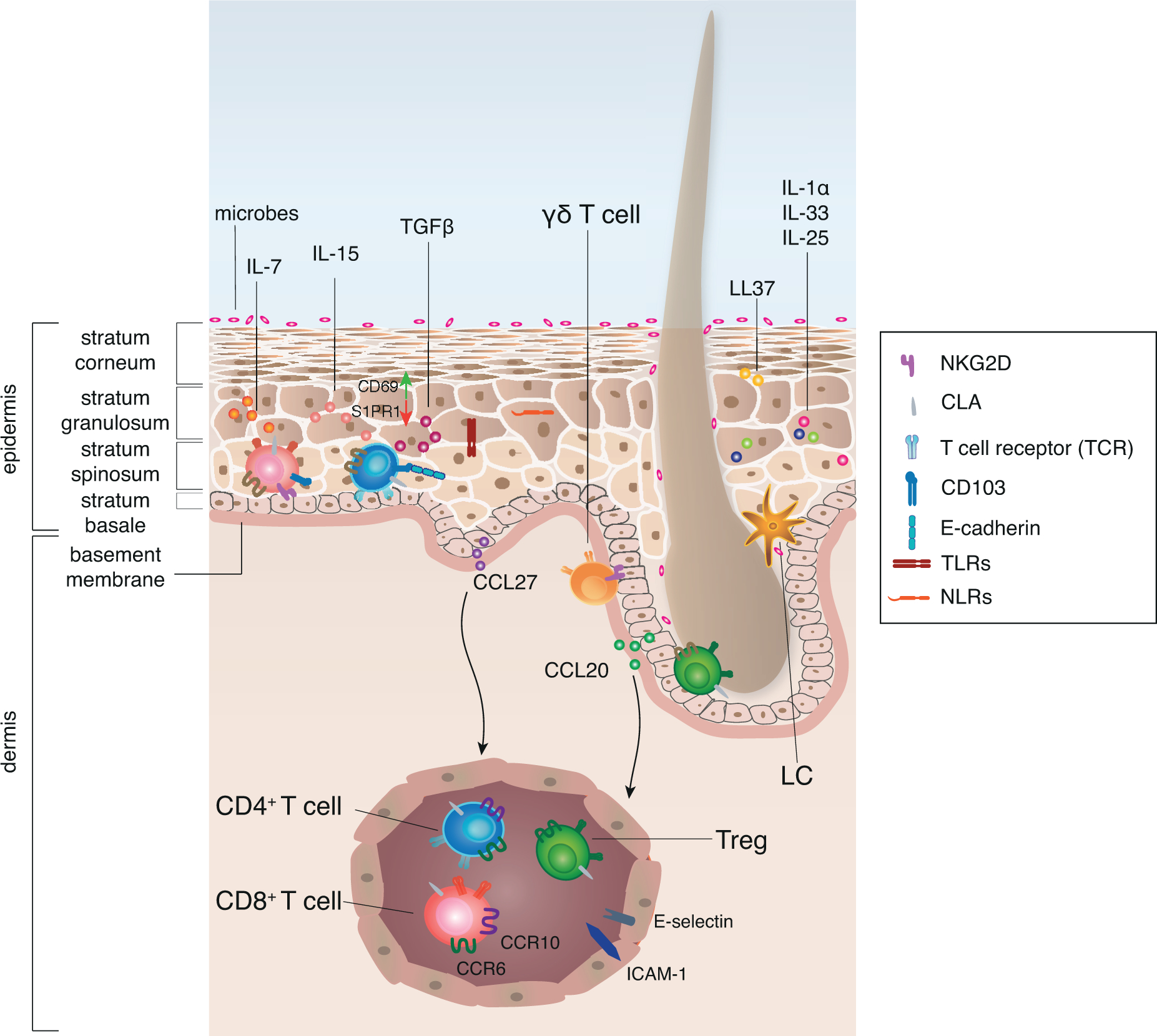
The human epidermis is mainly comprised of keratinocytes and can be divided into different layers. Keratinocytes function as sentinels that are equipped with TLRs and NLRs to sense danger signals, such as microbial components, chemicals or stress signals released upon damage, so called PAMPs and alarmins. Alarmins are produced by keratinocytes themselves and show anti-microbial properties, such as secretion of LL37. Additionally, keratinocytes are equipped with pre-stored cytokines, such as IL-1α, IL-25 and IL-33 that are released upon cell damage. The immune function of the epidermis is supported by specialized populations of antigen-presenting cells, CD1a+ Langerhans cells (LC), as well as innate lymphoid cells, and adaptive tissue-resident T cells. CCL20 produced by keratinocytes of the HF co-localizes LC and Treg and thus HF may serve as a niche for the induction of peripheral tolerance. Similarly, γδ T cells are often associated with adnexal structures.
The human skin provides a variety of survival and maintenance signals such as IL-7, IL-15 and TGF-β to support the maintenance of tissue-resident T cells that ensure quick response to infection. Keratinocyte-derived CCL27 and CCL20 allow for the recruitment of T cells from the blood that express CCR10 and CCR6 respectively to non-inflamed tissue. T cells that sense the chemokine gradient can extravasate through post-capillary venules mediated by E-selectin - CLA interaction and ICAM-1. Upon tissue entry T cells change their surface receptor expression, probably induced by tissue-derived signals. In order to maintain antigen-experienced T cells within the epidermis, downregulation of S1PR1 and upregulation of CD69 are supported by IL-15 and TGF-β. In addition, TGF-β induces upregulation of CD103 on T cells that allows them to interact with E-cadherin on keratinocytes, which supports the retention of resident memory T cells.
