Figure 2: Keratinocytes orchestrate damage response and its regulation in human skin.
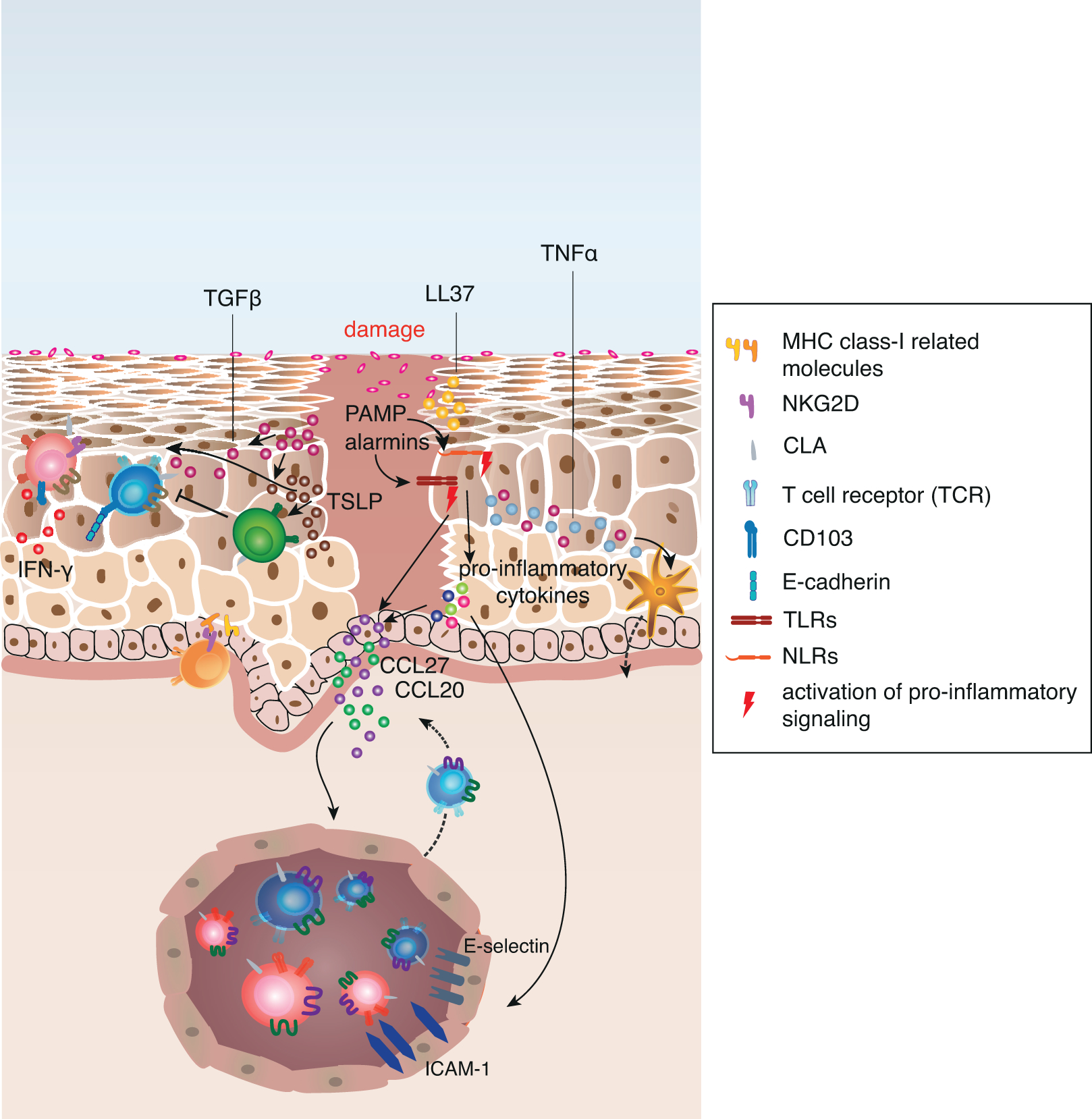
Upon tissue damage pre-stored cytokines are released from damaged cells and initiate immediate effector functions in adjacent cells. Sensing of microbial components and stress molecules (PAMPs and alarmins) by TLRs and NLRs result in the activation of pro-inflammatory signalling pathways such as NF-κB and IRF transcription. This leads to the increased production of pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-18, TNFα and type I interferons. Keratinocyte-derived TNFα activates Langerhans cells to migrate to skin-draining lymph nodes and activate naive or central memory T cells that upregulate CLA expression upon interaction with the LC. Additionally, the pro-inflammatory cytokines mediate the upregulation of chemokines and adhesion molecules on post-capillary venules as well as on adjacent keratinocytes, which promotes recruitment of circulating skin-tropic T cells from the blood.
In addition to these mechanisms, keratinocytes upregulate MHC class I-related gene products on their surface that engage NKG2D on CD8+ T cells and γ5 T cells. Pro-inflammatory cytokines also allow keratinocytes to directly activate antigen-experienced memory CD4+ T cells. Activated T cells in the tissue secrete pro-inflammatory cytokines, (e.g. IFN-γ), that in turn activate chemokine secretion by keratinocytes and increased alarmin and anti-microbial peptide (e.g. LL37) production.
TGF-β and TSLP secretion is increased upon tissue damage, and while both cytokines induce a variety of pro-inflammatory gene products and induce migration of LCs to skin-draining lymph nodes, they are essential to keep the inflammation local and support its resolution by directly signaling to Treg. TGF-β also promotes the local formation of resident memory T cells to provide quick response in case of a secondary antigen encounter.
