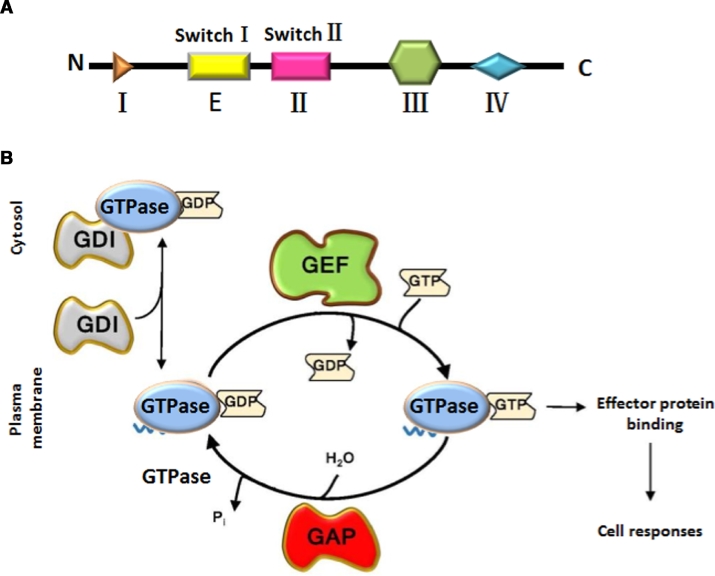
Fig. 1.
(A) Schematic diagram of the small GTPase protein sequence. (B) Regulation of small GTPase activity. GTPases cycle between their GTP-bound active and GDP-bound inactive form. They are activated by guanine-nucleotide exchange factors (GEFs), which remove GDP, thus enabling excess cellular GTP to bind. The binding of active GTPase to downstream effector proteins elicits cell responses. GTPase-activating proteins (GAPs), which increase the GTPase activity, are the off-switch. Inactive Rac is sequestered in the cytosol by guanine-nucleotide dissociation inhibitors (GDIs). Reproduced with permission from [78]. Copyright 2018 John Wiley & Sons, Inc.