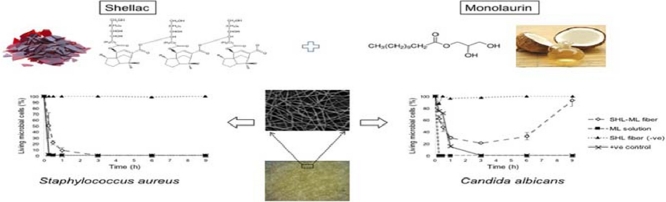
Graphical Abstract
The shellac nanofibers loaded with monolaurin were prepared by electrospinning technique based on a full factorial design. The obtained nanofibers exhibited antimicrobial activities against Staphylococcus aureus and Candida albicans over a period of time.
Keywords: Shellac, Monolaurin, Nanofibers, Factorial design, Wound dressing, Electrospinning
Abstract
The aim of this study was to elucidate the optimized fabrication factors influencing the formation and properties of shellac (SHL) nanofibers loaded with an antimicrobial monolaurin (ML). The main and interaction effects of formulation and process parameters including SHL content (35%–40% w/w), ML content (1%–3% w/w), applied voltage (9–27 kV) and flow rate (0.4–1.2 ml/h) on the characteristic of nanofibers were investigated through a total of 19 experiments based on a full factorial design with three replicated center points. As a result, the SHL content was the major parameter affecting fiber diameter. Another response result revealed that the SHL content would be also the most significant negative impact on amount of beads. An increase in the concentration of SHL leaded to a reduction in the amount of beads. From the results of characterization study, it was proved that ML might be entrapped between the chains of SHL during the electrospinning process exhibiting an excellent encapsulation. According to the response surface area, small (~488 nm) and beadless (~0.48) fibers were obtained with the SHL and ML contents of 37.5% and 1.1% w/w respectively, at the applied voltage of 18 kV and the flow rate of 0.8 ml/h. In addition, the results of the kill-kinetic studies showed that SHL nanofibers loaded with ML exhibited an excellent antibacterial activity against Staphylococcus aureus, while Escherichia coli was less affected due to the hydrophilic structure of the its outer membrane. ML also exerted an antifungal activity by reducing the number of Candida albicans colonies. Based on their structural and antimicrobial properties, SHL nanofibers containing ML could be potentially used as a medicated dressing for wound treatment.
1. Introduction
Wound is an injury which damages the skin, a protective barrier against the external environment. When a wound is exposed to the air, the scab, a crust of dried blood or serum, would be formed to cover the wound resulting in delayed wound healing [1]. Electrospun wound dressings work on the concept of moist wound healing which retains fluid contacting with wounds. The benefits of moist wound healing include inhibiting dry scab formation, decreasing the environment pH, affecting bacterial growth, shortening of the inflammatory phase of wound healing and providing an environment rich in enzymes, white blood cells and growth factors favorable for wound healing [2]. Due to their ultrafine network structures, electrospun nanofibers can be used for the replacement of extra cellular matrix (ECM) resulting in greatly rapid epithelialization and allowing the reduction of scar formation [[3], [4]]. The porous nature of nanofibers dressings also enhances oxygen and nutrients transferring to cells leading to an increased healing rate [5]. In addition, they can improve drug loading capacity, and release rate because of their high surface to volume ratio properties [6].
With the aim of being placed in the biological and physiological environment of a wound, nanofibers are mostly developed from biopolymers. SHL is a nontoxic natural polymer secreted by the lac insect, on some specific trees mainly in China, India and Thailand. There were few studies on the application of SHL in wound healing management, especially through electrospinning process [7]. Currently, SHL is widely used for enteric coating to protect drugs from acid degradation in the gastrointestinal tract and fruit coating to prevent microbial entry and water loss [[8], [9], [10]]. Due to the excellent film forming and protective properties, electrospun shellac nanofibers could be used as potential wound dressings.
Infection might cause a delay in wound healing. To prevent the multiplication of pathogens, an antimicrobial agent should be incorporated. Apparently, antibiotic resistance has become an increasing worldwide problem for infection treatment. Therefore, safe and effective antimicrobials which are not easily subject to resistance are greatly required. Among these simple antimicrobial compounds, natural lipids seem to be an achievable choice [11]. Fatty acids and their corresponding esters might have little or no toxicity and also exert antimicrobial activity. Isaacs, Litov and Thormar (1995) found that fatty acids and monoglycerides with medium chain lengths yielded more biologically active than long chain monoglycerides in killing viruses and bacteria [12]. Among medium chain fatty acids (C8 to C14), lauric acid (C12) has more antimicrobial activity than other fatty acids such as myristic acid (C14), capric acid (C10) or caprylic acid (C8) [13]. It could be found in human breastmilk, skin surface and other natural foods such as coconut oil and palm kernel oil [13]. ML, known as glycerol monolaurate, is a monoester form of lauric acid. It has many times greater antibacterial and antiviral activity than lauric acid. Tangwatcharin and Khopaibool (2012) revealed that minimum bactericidal concentrations (MBC) of lauric acid and ML were 3.2 mg/ml and 0.1 mg/ml, respectively [14]. The lower MBC value of ML indicated the stronger antimicrobial activity than that of lauric acid. ML has Generally Recognized As Safe (GRAS) status considered to be nontoxic [13]. It is approved in the US as a food emulsifier and has been used to control growth of pathogenic organisms and spoilage in food processing industries [15]. ML exhibits a broad-spectrum bactericidal activity against Grampositive bacteria from superficial skin infections and also has an antifungal effect by inhibiting spore germination and preventing the radial growth [[13], [16], [17], [18]]. Previous studies revealed that the topical use of ML could inhibit the growth of Staphylococcus aureus, a significant cause of skin and mucosal infections [18]. Besides the killing effect, ML might stabilize mucosal and skin surfaces, thus avoiding inflammation and further infection [18]. In addition to S. aureus, Streptococcus pyrogenes, Clostridium perfringens, Mycobacterium terrae, Listeria monocytogenes, Streptococcus agalactiae, and Groups C, F, and G streptococci could be killed by ML [[13], [18]]. Fungi, yeast, and protozoa were also reported to be inactivated by ML. Candida albicans, an opportunistic human yeast pathogen found in the oral pharynx, gut, genito-urinary tract and skin, was potentially affected by ML [19]. Additionally, the development of microbial resistance to ML was also considered rare to occur [18]. However, there are few studies exploring the possibility of ML as an alternative antimicrobial agent in the pharmaceutical dosage forms, including wound dressing.
The morphology of electrospun nanofibers and other related properties could be affected by various parameters which are broadly divided into formulation parameters, process parameters and ambient parameters [20]. Experimental design techniques have been applied to determine the main effects and interaction between parameters, and also to reduce development time and the numbers of experimental trials [21].
The aims of this research were to investigate which factors (SHL and ML concentrations, applied voltage and flow rate) and interaction effects between these factors would have the most impact on the morphology of SHL nanofibers loaded with ML as well as the interaction among these factors. The fabrication conditions were optimized by using a full factorial design with three replicated center points. Various instrumental analyses including powder X-ray diffraction, differential scanning calorimetry and FTIR spectroscopy were also employed in order to investigate the physicochemical characteristics of ML in SHL matrix.
2. Materials and methods
2.1. Materials
SHL (Lot No. 55) and ML (Lot No. 150520) were purchased from Mahachai Shellac Part., Ltd. (Samut Sakhon, Thailand) and Shanghai Terppon Chemical Co., Ltd., respectively. The following microbial strains obtained from Department of Medical Sciences, Thailand were performed in the antimicrobial assay: S. aureus DMST 8013, Escherichia coli DMST 4212 and C. albicans DMST 5815. The media including tryptic soy broth and agar (TSB, Lot No. VM229958 and TSA, Lot No. VM221159) were purchased from Merck KGaA (Germany) and sabouraud dextrose broth and agar (SDB, Lot No. VM331339 and SDA, Lot No. 3200237) were purchased from Merck KGaA (Germany) and Becton, Dickinson and Company (USA), respectively. Sterile powders of streptomycin (Lot No. 9/2015) and ketoconazole (Lot No. KT/14/08/18514) were obtained from General Drugs House Co., Ltd. and Greater Pharma Co., Ltd., respectively, and used as positive controls for antimicrobial test. Ethanol (95% v/v) used as a solvent for preparing spinning solutions was of reagent grade.
2.2. Physicochemical characterization of monolaurin loaded shellac films and fibers
The SHL-based polymeric films containing different concentrations of ML were initially prepared by dissolving definite amount of SHL (40% w/w) and varied amounts of ML in the range of 3%–15% w/w in 95% v/v ethanol under overnight stirring at room temperature followed by solvent evaporation. The highest level of ML loading in the obtained films was accordingly analyzed using powder X-ray diffraction (PXRD). Consequently, SHL nanofibers loaded with the maximum amount of incorporated ML were fabricated and further evaluated for the physicochemical characteristics using powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC) and Fourier transformed infrared spectroscopy (FTIR).
2.2.1. Powder X-ray diffraction (PXRD)
The crystalline or amorphous characteristic of the samples was evaluated using powder X-ray diffractometer (MiniFlex II, Rikaku, Japan). The scan was performed at 30 kV and 15 mA in the 2θ range of 5° to 40° with the speed of 4°/min using Cu Kα radiation (λ = 0.154 nm).
2.2.2. Differential scanning calorimetry (DSC)
The thermal characteristic of materials was investigated using differential scanning calorimeter (Sapphire, Perkin Elmer, Germany) under nitrogen gas flow with the rate of 20 ml/min. Samples were sealed in standard liquid aluminum pans and heated from 25 to 200 °C with the rate of 10 °C/min.
2.2.3. Fourier transformed infrared spectroscopy (FTIR)
The molecular behavior and interaction of ML in SHL matrix was examined using FTIR spectrophotometer (Nicolet Avatar 360, USA). During the process, each sample was ground and triturated with dry potassium bromide (KBr), and consequently compacted by hydraulic press machine to form pellet. The obtained pellet was placed in the sample holder. The scan was processed from 4000 to 400 cm−1 at 4 cm−1 resolution. The FTIR spectrum of each sample was obtained and analyzed using the OMNIC FTIR software.
2.3. Fabrication of monolaurin loaded shellac fibers through electrospinning process
2.3.1. Preparation and evaluation of shellac-monolaurin solutions
The solutions at selected SHL-ML ratios were prepared by dissolving finely ground SHL and ML in 95% v/v ethanol and then magnetically stirring for overnight at room temperature before adjusting to the desired content. In order to eliminate insoluble contamination, the homogenous solution was centrifuged at 8000 rpm for 30 min. The obtained solution was further subjected to study solution property, and fiber morphology after fabrication via electrospinning process.
The properties of SHL-ML solutions including viscosity, surface tension and conductivity were examined by rheometer (Malvern Kinexus, UK), drop shape instrument (FTA 1000, USA) and electrical conductivity meter (Extech EC500, USA), respectively.
2.3.2. Preparation and evaluation of shellac-monolaurin fibers
In this process, each SHL solution with ML was filled in a syringe linked with a high-voltage power supply. An electric field was performed between the metallic needle tip of the syringe and the collector. When the voltages were applied, nanofibers were then produced on the surface of a target drum wrapped with an aluminum sheet which stored at the controlled conditions (23–26 °C and 40%–60% RH). The morphology of the obtained fibers was investigated using scanning electron microscope (SEM) (LEO 1450 VP, UK). Based on SEM images, J MicroVision 1.2.7 software was utilized to measure the diameter of the fibers and the amount of beads. The influence of independent factors; formulation and process parameters including viscosity, surface tension, conductivity, applied voltage and feed rate, on the morphological characteristics of fibers were determined in order to find the significant factors for further design of experiment.
2.4. Experimental design
A 24 full factorial design with three replicates at the center point was performed in order to find the optimized significant independent factors for the multiple responses of fiber properties. The diameter of fibers and the amount of beads (bead-to-fiber ratio) were the observed responses. The critical variables and their levels considered in the design of experiment are shown in Table 1. The Design Expert 8.0.4 software (Stat-Ease Inc., USA) was used for the analysis of the resultant data. In addition, the optimum conditions for the fabrication of nanofibers with desired morphology were also determined.
Table 1.
Experimental range and levels of independent parameters.
| Parameters | Symbol | Variable levels |
||
|---|---|---|---|---|
| Low (−1) | Central point (0) | High (+1) | ||
| SHL content [% w/w] | X1 | 35.0 | 37.5 | 40.0 |
| ML content [% w/w] | X2 | 1.0 | 2.0 | 3.0 |
| Applied voltage [kV] | X3 | 9.0 | 18.0 | 27.0 |
| Flow rate [ml/h] | X4 | 0.4 | 0.8 | 1.2 |
2.5. Time–kill kinetics
Time–kill assays were performed in order to study the pharmacodynamics of antimicrobial agents, showing a profile of antimicrobial activity over time. Prior to the kinetic test, S. aureus and E. coli were cultured in TSB media. Whereas C. albicans were cultured in SDB media. After overnight incubation at 37 °C, the density of inoculation was further diluted to 104 CFU/ml. For the kinetic test, 30 min UV-sterilized SHL nanofibers loaded with ML and ML in a solution form were added in the media broth to give the same final concentration of ML (2 mg/ml). SHL fibers without ML were used as the negative control, while streptomycin and ketoconazole were used as positive controls for antibacterial and antifungal test, respectively. All tubes were shaken and incubated at 37 °C. At different time points (0, 15, and 30 min, 1, 3, 6 and 9 h), 100 µl of each sample was taken and spread on a media agar. All plates were then incubated for 24 h at 37 °C. The number of colonies was counted and calculated for the percentage of living microbial cells as following equation.
| (1) |
where C0 and Ct are the number of colonies at initial time (before adding the sample) and each time point, respectively.
3. Results and discussion
3.1. Evaluation of physical state and compatibility of the components in monolaurin loaded shellac matrix
The physicochemical characterizations of ML loaded SHL matrix including crystallinity property, thermal characterization and molecular behavior and interaction were determined by using PXRD, DSC and FTIR as described below.
3.1.1. Powder X-ray diffractometry (PXRD)
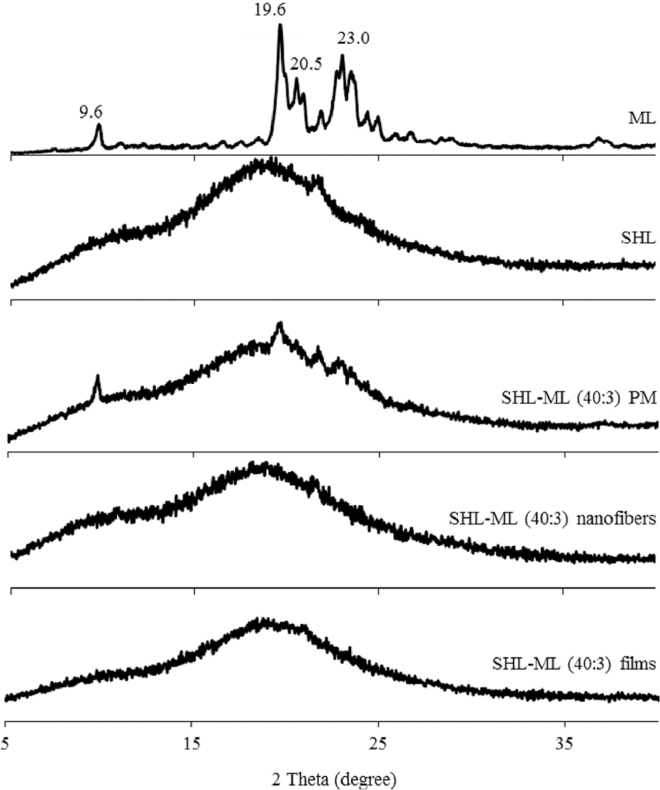
The PXRD patterns of SHL powder, ML powder, their physical mixture, nanofibers and films in the weight ratio of 40:3 are illustrated in Fig. 1. The crystalline peaks as indicated by the diffraction peaks at 2θ values of 9.6°, 19.6°, 20.5° and 23.0° were clearly observed in the PXRD pattern of ML powder while SHL powder was in amorphous state as presented in a halo PXRD pattern. After encapsulation of ML in SHL nanofibers or films, the characteristic crystalline peaks of ML were absent assuming that ML might be entrapped in the SHL matrix. It was also noted that fast drying rate during electrospraying was not the cause of disappearance of ML diffraction peak (amorphization) since slow evaporation during the film casting also demonstrated the similar PXRD pattern as that obtained from electrospraying. In this case, the ML molecules should be molecularly dispersed among the polymer chains of SHL, resulting in the disappearance of crystalline structure.
Fig. 1.
Powder X-ray diffraction patterns of SHL, ML, their physical mixture, nanofibers and films in the weight ratio of 40:3.
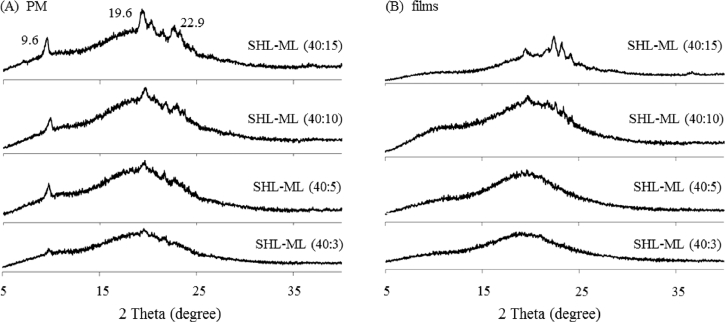
The SHL films containing various amounts of ML were developed aiming to investigate the highest level of incorporated ML. Based on Fig. 2A, the diffraction peaks due to crystalline ML were clearly observed in the PXRD patterns of all SHL-ML physical mixtures, especially at high fraction of ML. However, the peak intensity due to ML in PXRD patterns of SHL-ML films (Fig. 2B) became obviously decreased as compared to that of the corresponding physical mixtures. According to the PXRD profiles of SHL-ML films at the ratios of 40: 5 and 40: 3, the diffraction peaks completely disappeared and changed to halo pattern, suggesting the complete encapsulation or monomolecular dispersion of ML into SHL matrix films at these ratios. Thus, the suitable ratio of SHL to ML should be 40: 5 in which ML could be completely loaded in SHL nanofibers. Nevertheless, after preliminary investigation by electrospinning process, nanofibers containing 40: 5 weight ratio of SHL to ML could not be performed because of tackiness. Therefore, the combination of SHL with ML at the ratio of 40: 3 was found to be the maximum loading amount of ML.
Fig. 2.
Powder X-ray diffraction patterns of (A) SHL-ML physical mixtures with different amounts of ML and (B) their corresponding films.
3.1.2. Differential scanning calorimetry (DSC)
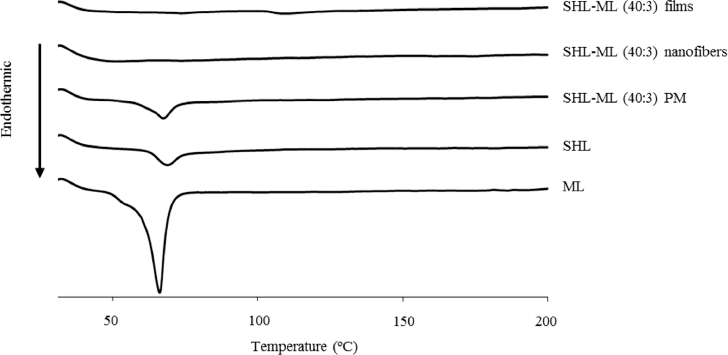
DSC is one of the common tools for studying the interaction between the active drug and excipients and also assessing the possible incompatibilities which is significantly involved in the dosage form development [22]. In this study, DSC was employed to study the interaction between SHL and ML. The DSC thermograms of SHL, ML and their corresponding samples are illustrated in Fig. 3. Based on the DSC curve of ML, a large endothermic peak was obtained at about 66 °C indicating the melting temperature of ML. Meanwhile, pure SHL displayed the melting peak at about 69 °C which was almost the same position as ML. The physical mixture showed the broad endothermic peak, presumably due to the overlapped melting peaks of ML and SHL, at 68 °C. However, the films and nanofibers demonstrated the different results as compared to those found in the physical mixture. The loss of endothermic peaks of ML and SHL was obviously observed in the DSC thermograms of the films and nanofibers which suggested the strong interaction between SHL and ML. The ML crystals might be changed from crystal state to molecular level and interacted with polymer structure of SHL during processing which was in accordance to the XRD results.
Fig. 3.
DSC thermograms of SHL, ML, their physical mixture, nanofibers and films in the weight ratio of 40:3.
3.1.3. Fourier transform infrared spectroscopy (FTIR)
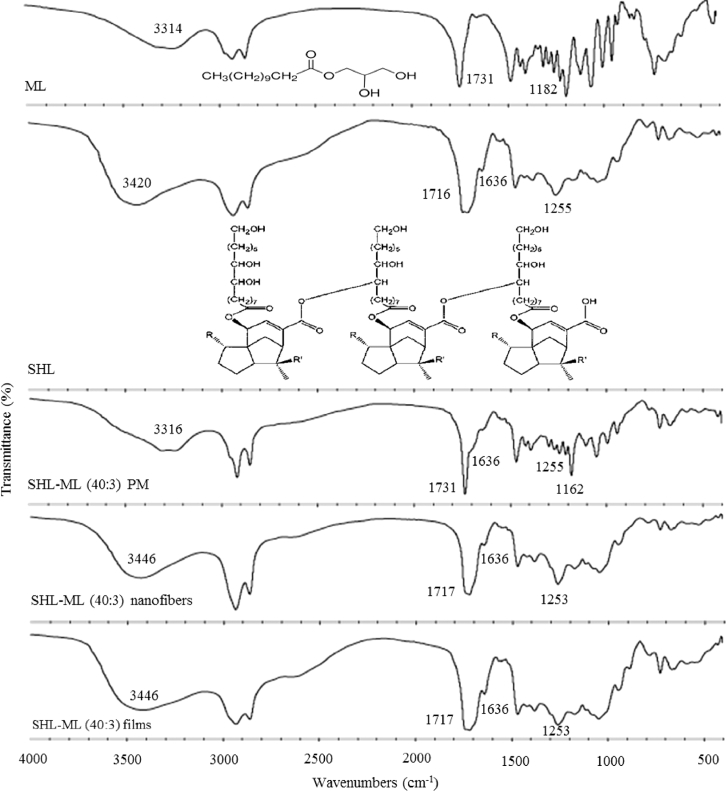
In order to provide more insight into the chemical interaction between components, FTIR was used for studying the molecular behavior of ML in SHL matrix. As illustrated in Fig. 4, ML showed a broad peak with the maxima around 3314 cm−1 which was assigned as O—H stretching of the hydroxyl groups at the glycerol backbone while the peaks at 1731 and 1182 cm−1 were attributed to carbonyl (C O) stretching and C—O stretching of lauryl ester, respectively [23]. SHL also demonstrated the peak due to O—H stretching of hydroxyl groups and C O stretching but in the different positions of 3420 cm−1 and 1716 cm−1, respectively. Additionally, the peaks assigned to alkenyl (C C) and C—O stretching band were observed at 1636 and 1255 cm−1, respectively [24]. For the physical mixture, the superimposed spectrum between SHL and ML was observed. However, the FTIR spectrum of ML loaded SHL fibers was clearly changed as compared to that of the physical mixture. After the incorporation of ML into SHL fibers, the shift of broad O—H bands of ML (3314 cm−1) and SHL (3420 cm−1) to the maxima around 3446 cm−1 was observed while the peaks assigned to C O stretching of ML (1731 cm−1) and SHL (1716 cm−1) were shifted to 1717 cm−1. Additionally, the peak intensity of ML was clearly decreased after loading into the SHL fibers. Besides, a characteristic peak assigned to C C stretching (1636 cm−1) of SHL in SHL-ML fibers was not changed which suggested that only some functional groups of SHL was involved in the interaction. Similar results were observed in the FTIR spectrum of SHL-ML films and therefore confirmed the specific interaction between SHL and ML after incorporation into the matrix of either SHL fibers or films. In this case, the intermolecular hydrogen bonding among the hydroxyl groups and carbonyl groups of SHL and ML might be a possible explanation.
Fig. 4.
FTIR spectra of SHL, ML, their physical mixture, nanofibers and films in the ratio of 40: 3.
3.2. Evaluation of SHL-ML solution properties
The solution property might play a significant role in the electrospinning process affecting the morphology of the obtained nanofibers. During the electrospinning process, the solution was ejected from the needle tip depending on the stretching capacity of the solution which would be influenced by conductivity, viscosity and surface tension [20]. Obviously, as illustrated in Table 2, the increased concentration of SHL led to a remarkable increase in viscosity, a slight decline in conductivity, but no significant change in surface tension. The viscosity was directly related to SHL content. As the concentration of SHL was increased, the amount of polymer chain entanglement was also increased resulting in a significant elevation in viscosity [20]. Generally, SHL is a non-ionic polymer with dielectric constant (ε) of 3.6 [25]. When the SHL content was increased, the electrical conductivity of a solution seemed to decline as a result of the decreased charge carrier mobility. Meanwhile, the surface tension of a solution was not significantly different. This could be explained by the dispersed distribution of SHL molecules in the solution which was not projected to the surface.
Table 2.
Effect of SHL and ML concentrations on the solution properties.
| SHL content (% w/w) | ML content (% w/w) | Solution properties |
||
|---|---|---|---|---|
| Viscosity (mPa⋅s) | Conductivity (µS) | Surface tension (mN/m) | ||
| 35.0 | 1.0 | 454.2 | 26.41 | 2.24 |
| 35.0 | 2.0 | 335.4 | 26.33 | 2.18 |
| 35.0 | 3.0 | 251.5 | 26.29 | 2.16 |
| 37.5 | 1.0 | 676.7 | 23.75 | 2.20 |
| 37.5 | 2.0 | 561.9 | 23.27 | 2.21 |
| 37.5 | 3.0 | 411.8 | 23.05 | 2.13 |
| 40.0 | 1.0 | 750.8 | 22.23 | 2.24 |
| 40.0 | 2.0 | 705.3 | 20.97 | 2.18 |
| 40.0 | 3.0 | 643.4 | 20.64 | 2.16 |
In addition, the introduction of ML into the SHL solutions could also affect the solution properties. When the content of ML was increased, the solution tended to have a slight reduction in viscosity which was presumably owing to the change in chain conformation of SHL. As discussed earlier, ML could be entrapped between the chains of SHL during the process of electrospinning, thus reducing the entanglement of SHL chains and resulting in a slight decreased in viscosity. A slight decrease in conductivity might be induced by an increase in the amount of ML due to the non-ionic property of ML [26]. However, the surface property of a solution was not changed after incorporating ML in the same manner as described above.
3.3. Experimental design
The morphology of electrospun nanofibers could be influenced by various parameters including formulation parameters, process parameters and ambient parameters [20]. As mentioned earlier, the solution property could affect the stretching capacity of the solution leading to the change of fiber morphology. Besides, studying all of the parameters in a single framework simultaneously seemed to be impossible and therefore needed the experimental design to reduce the number of experimental trials [21]. In this work, a two-level full factorial design along with three replicates at the center point was adopted in order to evaluate all possible combinations of parameter levels providing an accurate interpretation, along with an experimental error estimate [21]. Referring to the results obtained from preliminary experiments, the most significant factors affecting the morphology of electrospun nanofibers were the contents of SHL (X1) and ML (X2), applied voltage (X3) and flow rate (X4). Meanwhile, some variables might be held constant. In this procedure, the distance between the tip and collector was maintained at 20 cm. The process of electrospinning was conducted at the controlled conditions (23–26 °C and 40%–60% RH). In addition, the desired level of each factor was selected. Due to preliminary investigation, the concentration of SHL should be between 35% and 40% w/w. With the reduced content of SHL below 35% w/w, polymeric beads were generated instead of continuous fibers, and with the increased amount of SHL above 40% w/w, the nanofibers could not be fabricated resulting from the hard ejection of polymer solution. The optimum concentration of ML was in a range of 1% to 3% w/w. At a content of ML above 3% w/w, the loaded nanofibers found to be liquefied. In case of processing parameters, the effective range of applied voltages for the fabrication process was between 9 and 27 kV. The high voltages (over 27 kV) might produce electrical arcs, while the drawing capacity of polymer solution from the tip was poor with the applied voltages below 9 kV. The appropriate flow rate was in a range of 0.4 to 1.2 ml/h.
3.3.1. Effects of the independent parameters on the morphology of SHL-ML nanofibers
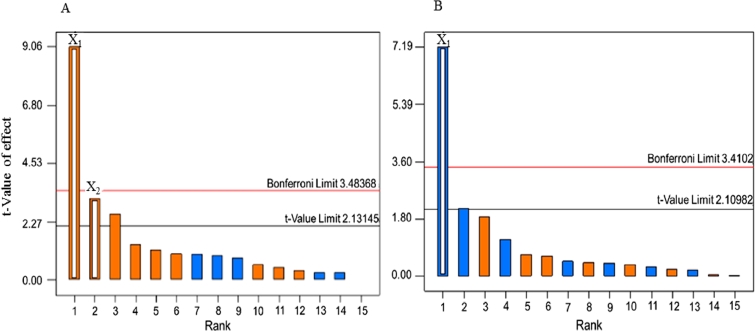
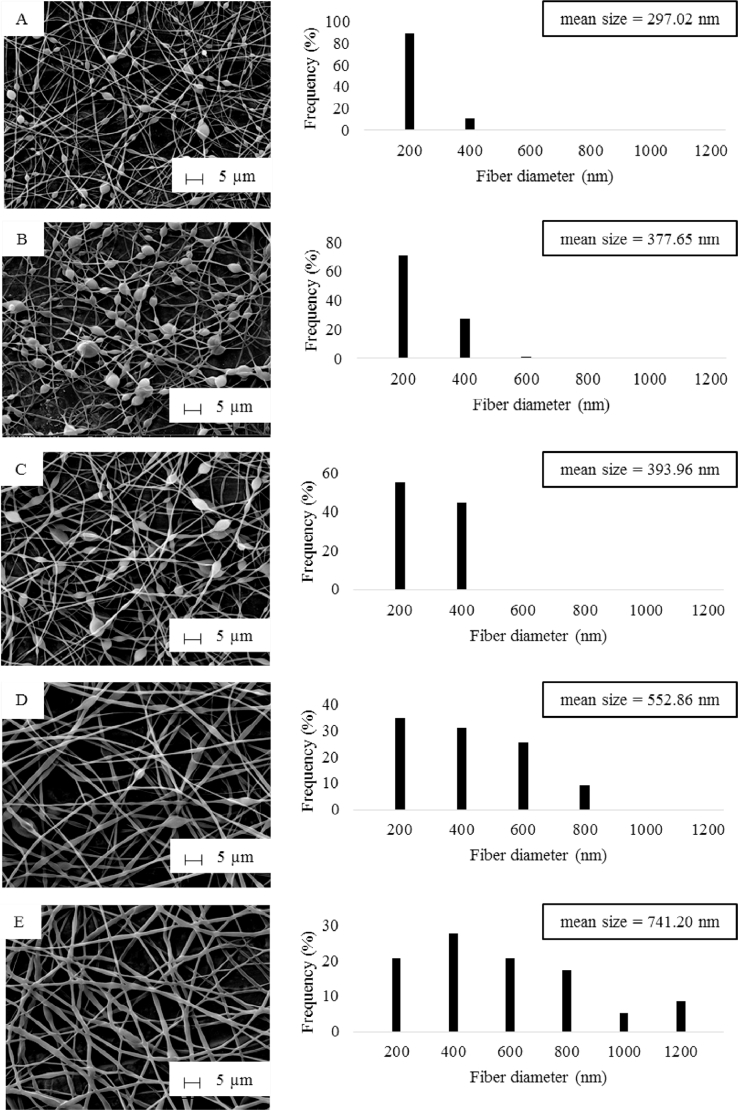
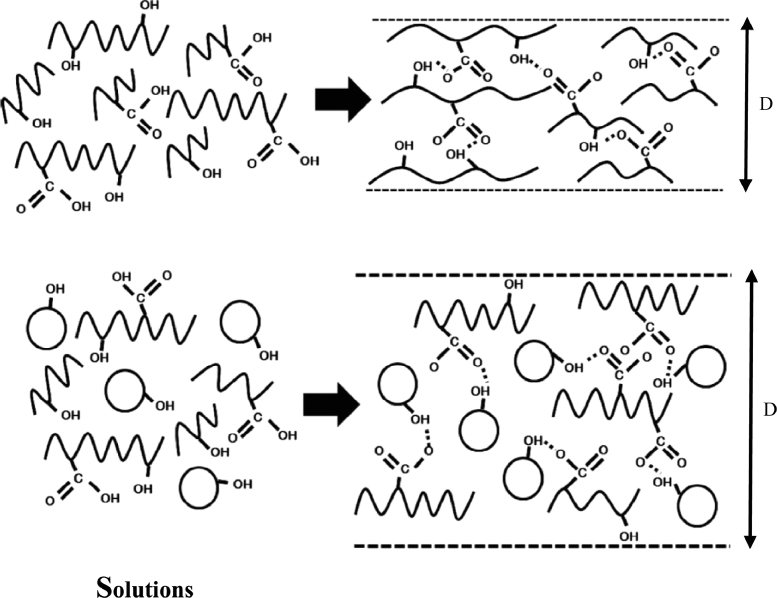
A series of 19 experiments based on a 24 full factorial design with three replicated center points was performed (Table 3). Pareto charts were presented via a series of ordered bar graphs in order to determine the most significant factors and their interactions. In the presented plot, there were two different t limits including the Bonferroni-corrected t and the standard t. The factor effects might become certainly significant as presented above the Bonferroni limit. The effects were possibly significant as displayed above the t value limit while the influences seemed to be not significant as shown below the t value limit [27]. As demonstrated in Fig. 5A and Fig. 6, Pareto charts and the diameter distributions of nanofibers at different concentrations of SHL and ML revealed that the SHL content (X1) had a tremendous effect on the diameter of fibers while the ML level (X2) was the minor factor. For examples, electrospun fibers prepared from 40% w/w SHL (Fig. 6D, 6E) indicated the larger diameter as compared to those prepared from 35% w/w SHL with the same concentration of ML (Fig. 6A, 6E). Similarly, the electrospun fibers prepared from a higher concentration of ML (Fig. 6A, 6B) demonstrated the larger fiber size as compared to those prepared from a lower concentration of ML (Fig. 6D, 6E) although less extent was observed. As mentioned earlier (Table 2), the increased concentration of SHL resulted in the significant increase of viscosity and the slight decrease of conductivity leading to a reduction in the stretching capacity of the solution. As a result, nanofibers with large diameters might be obtained [20]. Another factor affecting the fiber diameter was the concentration of ML. Based on theoretical expectation, an increase in the viscosity of electrospun solution contributes to an increase in the resultant fiber diameter due to a decrease in the stretching capacity during the electrospinning process [20]. By contrast, the fabricated nanofibers appeared to be thicker with the presence of increased ML, although the reduced viscosity was clearly indicated. In this case, the influence of viscosity as well as the stretching capacity might be presumably dominated by another factor. As explained in the section 3.1, the possible interaction between SHL and ML was observed. We therefore assumed that the interaction of ML seemed to be the likely cause of the reduced stretching ability of solutions and thus increasing the fiber diameter. ML might interact with the SHL by intercalating among the polymeric chains and thus impeding the elongation or stretching capacity (as postulated in Fig. 7). Additionally, the slight decrease of solution conductivity as increasing ML contents also reduced the charge density of electrospun solutions leading to the impaired elastic force within the jet [20]. Nevertheless, the impacts of applied voltage and feed rate on the fiber morphology tended to be not important as apparently displayed below the t value limit (Fig. 5).
Table 3.
Full factorial design and experimental responses for each design point.
| Run | X1 | X2 | X3 | X4 | Fiber diameter (Y1) [nm] | Bead amount (Y2) |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | 297 | 0.76 |
| 2 | +1 | −1 | −1 | −1 | 554 | 0.05 |
| 3 | −1 | +1 | −1 | −1 | 378 | 1.04 |
| 4 | +1 | +1 | −1 | −1 | 741 | 0.02 |
| 5 | −1 | −1 | +1 | −1 | 309 | 0.80 |
| 6 | +1 | −1 | +1 | −1 | 774 | 0.03 |
| 7 | −1 | +1 | +1 | −1 | 448 | 1.04 |
| 8 | +1 | +1 | +1 | −1 | 695 | 0.02 |
| 9 | −1 | −1 | −1 | +1 | 315 | 1.20 |
| 10 | +1 | −1 | −1 | +1 | 714 | 0.16 |
| 11 | −1 | +1 | −1 | +1 | 549 | 0.43 |
| 12 | +1 | +1 | −1 | +1 | 830 | 0.03 |
| 13 | −1 | −1 | +1 | +1 | 357 | 0.96 |
| 14 | +1 | −1 | +1 | +1 | 783 | 0.03 |
| 15 | −1 | +1 | +1 | +1 | 544 | 0.42 |
| 16 | +1 | +1 | +1 | +1 | 888 | 0.03 |
| 17 | 0 | 0 | 0 | 0 | 394 | 0.75 |
| 18 | 0 | 0 | 0 | 0 | 411 | 0.65 |
| 19 | 0 | 0 | 0 | 0 | 335 | 0.78 |
Fig. 5.
Pareto charts of the response values of (A) nanofiber diameter and (B) bead amount.
Fig. 6.
SEM images and diameter distribution of SHL-ML nanofibers at the weight ratio of (A) 35: 1, (B) 35: 3, (C) 37.5: 2, (D) 40: 1 and (E) 40: 3.
Fig. 7.
Schematic diagram representing the influence of ML on fiber diameter.
The occurrence of beads on the electrospun fibers is considered as defect. As indicated by Pareto chart in Fig. 5B, only the content of SHL (X1) had a remarkable impact on the appearance of beads. The number of beads had a tendency to decrease with increasing concentration of SHL while the smooth fibers were obtained from 40% w/w SHL and 3% w/w ML (Fig. 6). Based on Table 2, an increase in the viscosity of SHL might lead to the high interaction between the polymers and solvent molecules. The aggregation probability of the solvent molecules was thus reduced. As a result, the beaded fibers were gradually changed to smooth fibers [20]. In order to predict the impact of independent factors on the diameter of fiber (Y1) and the amount of bead (Y2) represented by bead-to-fiber ratio, mathematical models were performed as presented in the following equations. The Equation 2 and 3 referred to actual factors with R2 values of 0.7480 and 0.7527, respectively indicating the good fit of the model.
| (2) |
| (3) |
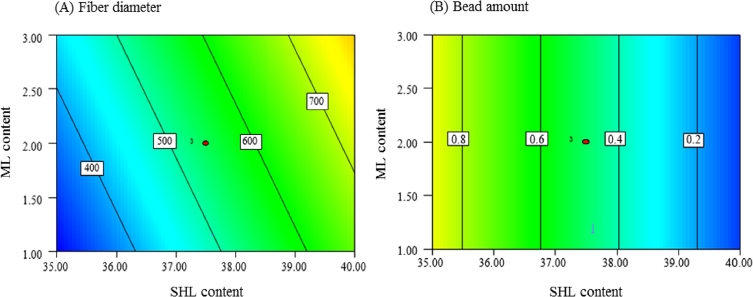
According to the results of ANOVA shown in Table 4, the prediction of the models would be reliable referring to the P values of the models which were less than 0.05. Moreover, the lack of fit was not significant with P values of 0.1157(Y1) and 0.0827(Y2) indicating that these models were suitable. The coefficients represented the magnitude and direction of the factor effects. As presented in Table 4, SHL content (X1) was the most influential parameter on the nanofiber diameter and bead amount with positive and negative relationships respectively, corresponding to the results from Pareto diagrams (Fig. 5) and two-dimensional contour plots (Fig. 8) which were also displayed in order to estimate the effect of each factor on the responses. Based on Fig. 8A, the small fibers could be generated from the low amounts of SHL and ML. Meanwhile, the beadless fibers might be obtained from the high concentration of SHL regardless of the amount of SHL as depicted in Fig. 8B.
Table 4.
Results of ANOVA for different responses.
| Responses | Coefficients | P value | Std. Dev. | % CV | Mean value | R2 | F value |
|---|---|---|---|---|---|---|---|
| Y1 (nanofiber diameter) | 106.87 | 19.68 | 542.89 | 0.7480 | |||
| X1 | 173.81 | <0.0001 | |||||
| X2 | 60.69 | 0.0373 | |||||
| Intercept | 542.89 | ||||||
| Model | <0.0001 | 23.74 | |||||
| Lack of fit | 0.1157 | 8.06 | |||||
| Y2 (bead amount) | 0.22 | 45.08 | 0.48 | 0.7527 | |||
| X1 | −0.39 | <0.0001 | |||||
| Intercept | 0.48 | ||||||
| Model | <0.0001 | 51.73 | |||||
| Lack of fit | 0.0827 | 11.52 |
Fig. 8.
Two-dimensional contour plots presenting the effects of SHL and ML contents on (A) fiber diameter and (B) bead amount.
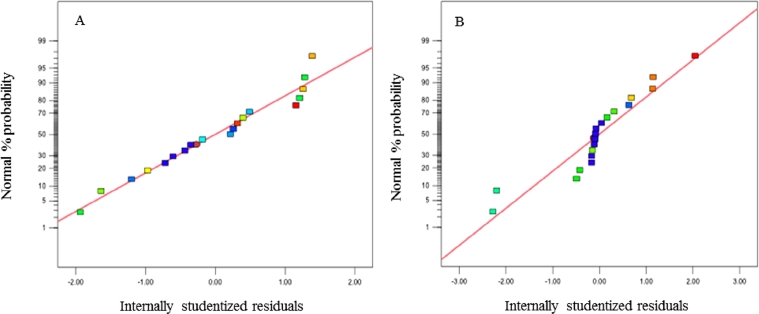
The normal probability plots of residuals are illustrated in order to explain the validity of the obtained data. Referring to Fig. 9A and 9B, the residuals seemed to fit a straight line indicating that the errors were normally distributed; thus, the data were acceptable.
Fig. 9.
Normal probability plots of nanofiber (A) diameter and (B) bead amount.
3.3.2. Optimization of SHL-ML nanofibers
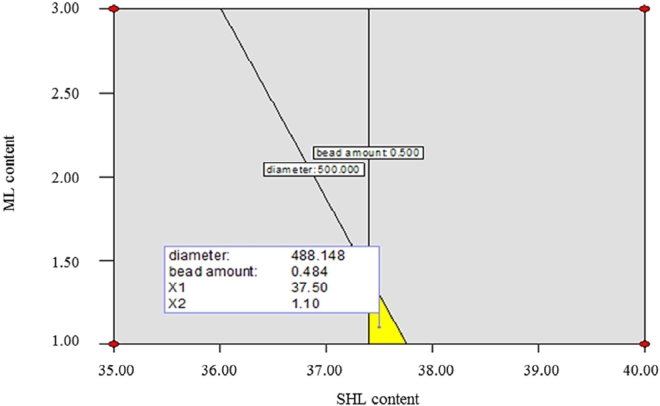
The graphical model was presented with the aim of exploring the optimal region leading to the fabrication of the desired nanofibers. There are two types of the represented graph which are generally displayed as the three-dimensional response surface and the contour plot illustrated by the plane surface [28]. In this study, an overlay contour plot was depicted as shown in Fig. 10. The chosen desirable ranges of fiber diameter and bead-to-fiber ratio were 300–500 nm and 0–0.5, respectively. Based on the overlay plot, the example of the optimum conditions for the fabrication of SHL-ML nanofibers with thinner diameter (~488 nm) and beadless (~0.48) was attained at the SHL and ML contents of 37.5% and 1.1% w/w respectively, with the applied voltage of 18 kV and the flow rate of 0.8 ml/h.
Fig. 10.
Overlay plot for optimization of SHL nanofibers loaded with ML.
3.4. Time–kill kinetic study
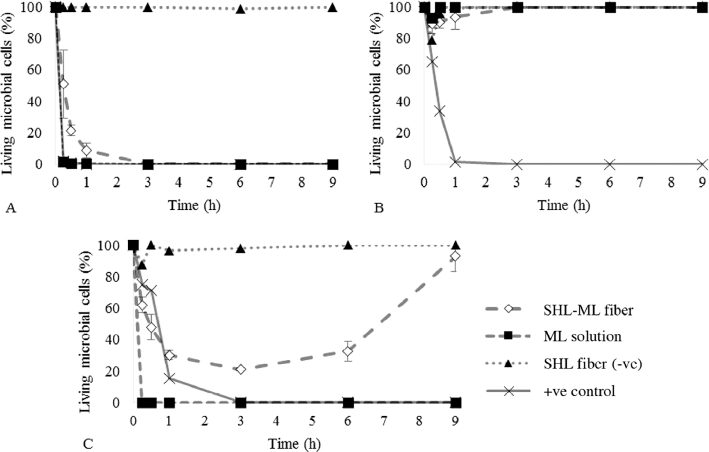
In this work, time–kill kinetic study was carried out in order to determine the pharmacodynamics of ML, providing a profile of antimicrobial effect against S. aureus, E. coli and C. albicans which represented Gram-positive and Gram-negative bacteria and fungi, respectively, at different time periods. The percentage of living cells of S. aureus, E. coli and C. albicans over a period of time was presented in Fig. 11. With regard to Fig. 11A, the number of S. aureus exposed to SHL-ML nanofibers gradually decreased as compared to those treated with ML solution which provided immediate killing potential, estimating that the release of ML from SHL nanofibers was dependent on the solubility of SHL in liquid media. Thus, the antibacterial action of SHL-ML nanofibers tended to be relatively sustained. The mode of action was previously reported. ML, a lipophilic compound, might be accumulated into the membrane bilayer, and consequently disintegrates the microbial membrane by fluidizing the lipids and phospholipids in the envelope of the organism, changing of the hydrogen bonding and the dipole–dipole interaction between acyl chains exerting bactericidal effects [14].
Fig. 11.
Kill kinetics of nanofibers for (A) S. aureus, (B) E. coli and (C) C. albicans over a period of 9 h.
Nevertheless, many studies reported that E. coli was found to be less affected by ML [29]. The difference in the killing effects of lipids against Gram-negative bacteria might depend on the differences in the outer membrane. Based on the structure of E. coli, the external membrane consists of proteins and lipopolysaccharides (LPS) which are composed of the O-polysaccharide chains demonstrating a hydrophilic surface property. Therefore, lipids, which are hydrophobic molecules, could have difficulty in entering the bilayer, and be hardly diffused in the cytoplasm [[30], [31]]. This was consistent with the result as shown in Fig. 11B. Both of SHL nanofibers loaded with ML and ML solution did not display activity against E. coli. The number of living cells was significantly increased over time.
As mentioned before, ML also has an antifungal effect by inhibiting spore germination and preventing the radial growth [17]. Fig. 11C demonstrated that some of C. albicans were killed by the ML released from fibers. However, after a period of 3 h, the amount of C. albicans then slightly increased, while the number of C. albicans exposed to ML in a solution form obviously declined without re-increasing. This might be explained by the effect of pH media on the solubility of SHL. According to the study of Limmatvapirat et al. [32], SHL with a pKa of 6.9–7.5 could be dissolved at pH above 7. Therefore, the solubility limitation of SHL in SDB media, which has an approximate pH value of 5.6, might occur. The release of ML from SHL carrier seemed to be incomplete which was due to the low pH of the liquid medium. During the initial period of exposure, only some of ML, especially at the surface of nanofibers was rapidly released and partially eradicated the C. albicans while most of ML was expected to be trapped in the SHL matrix and slowly released which was considered as the prolonged exposure time. As a result, the concentration of ML was not enough to inhibit the regrowth of living microorganisms after a 3-h exposure to SHL-ML nanofibers. Thus, the application of SHL-ML nanofibers in chronic wound management might be effective due to the predominant alkaline pH of chronic wounds (7.1–8.9), while treatment of acute wounds, which have low pH values of 5–6, seems to be restricted [33].
4. Conclusion
SHL nanofibers loaded with ML could be developed for wound dressing application in order to prevent the delayed wound healing resulting from microbial infection. The main and interaction effects of factors on the morphology of nanofibers were investigated by using a full factorial design with three replicated center points. According to the results, the SHL content was the major parameter affecting the fiber diameter, while the loaded ML content might be the minor parameter. The study of parameters on bead amount was evaluated revealing that the SHL content was the most significant negative impact on bead amount. The optimum conditions for the fabrication of small and beadless nanofibers were studied. The desired fibers could be obtained at the SHL and ML concentrations of 37.5% and 1.1% w/w respectively, with the applied voltage of 18 kV and the flow rate of 0.8 ml/h. Moreover, time–kill assays were performed in order to study the pharmacodynamics of antimicrobial agents, showing a profile of antimicrobial activity over time. The results showed that SHL nanofibers loaded with ML provided an excellent antibacterial activity against S. aureus whereas E. coli was less affected. ML also exhibited antifungal effect against C. albicans. However, the release of ML might depend on the solubility of SHL carrier. Therefore the effect of pH in wound environment was considerable. The application of SHL-ML nanofibers in chronic wound management might be effective due to the predominant alkaline pH of chronic wounds, while treatment of acute wounds, which have low pH values, seems to be restricted.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors acknowledge the financial support received from Silpakorn University Research and Development Institute. The study was also supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (2559A11462006); and Faculty of Pharmacy, Silpakorn University.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Abdelrahman T., Newton H. Wound dressings: principles and practice. Surgery. 2011;29:491–495. [Google Scholar]
- 2.Stashak T.S., Farstvedt E., Othic A. Update on wound dressings: indications and best use. Clin Tech Equine Pract. 2004;3:148–163. [Google Scholar]
- 3.Engel E., Michiardi A., Navarro M., Lacroix D., Planell J.A. Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol. 2008;26:39–47. doi: 10.1016/j.tibtech.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Pelipenko J., Kocbek P., Govedarica B., Rošic R., Baumgartner S., Kristl J. The topography of electrospun nanofibers and its impact on the growth and mobility of keratinocytes. Eur J Pharm Biopharm. 2013;84:401–411. doi: 10.1016/j.ejpb.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Unnithan A.R., Gnanasekaran G., Sathishkumar Y., Lee Y.S., Kim C.S. Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing. Carbohydr Polym. 2013;102:884–892. doi: 10.1016/j.carbpol.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 6.Hu X., Liu S., Zhou G., Huang Y., Xie Z., Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Thammachat T., Sriamornsak P., Luangtana-Anan M., Nunthanid J., Limmatvapirat C., Limmatvapirat S. Preparation and characterization of shellac fiber as a novel material for controlled drug release. Adv Mat Res. 2010;152–153:1232–1235. [Google Scholar]
- 8.Okamoto M.Y., Ibanez P.S. Final report on the safety assessment of shellac. J Am Coll Toxicol. 1986;5:309–327. [Google Scholar]
- 9.Alzahrani H., Bedir Y., Al-Hayani A. Efficacy of shellac, a natural product, for the prevention of wet gangrene. J Int Med Res. 2013;41:795–803. doi: 10.1177/0300060513483391. [DOI] [PubMed] [Google Scholar]
- 10.Porter S.C. In: Pharmaceutical dosage forms: tablet. Lieberman H.A., editor. Marcel Dekker Inc.; New York: 1990. Coating of pharmaceutical dosage forms; pp. 77–160. [Google Scholar]
- 11.Thormar H., Hilmarsson H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem Phys Lipids. 2007;150:1–11. doi: 10.1016/j.chemphyslip.2007.06.220. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs C.E., Litov R.E., Thormar H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. Nutr Biochem. 1995;6:362–366. doi: 10.1016/0955-2863(95)80003-u. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman S., Enig M.G., Preuss H.G. A review of monolaurin and lauric acid: natural virucidal and bactericidal agents. Altern Complement Ther. 2006;12:310–314. [Google Scholar]
- 14.Tangwatcharin P., Khopaibool P. Activity of virgin coconut oil, lauric acid or monolaurin in combination with lactic acid against Staphylococcus aureus. Southeast Asian J Trop Med Public Health. 2012;43:969–985. [PubMed] [Google Scholar]
- 15.Nobmann P., Smith A., Dunne J., Henehan G., Bourke P. The antimicrobial efficacy and structure activity relationship of novel carbohydrate fatty acid derivatives against Listeria spp. and food spoilage microorganisms. Int J Food Microbiol. 2009;128:440–445. doi: 10.1016/j.ijfoodmicro.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Carpo B.G., Verallo-Rowell V.M., Kabara J.J. Novel antibacterial activity of monolaurin compared with conventional antibiotics against organisms from skin infections: an in vitro study. J Drugs Dermatol. 2007;6:991–998. [PubMed] [Google Scholar]
- 17.Rihakova Z., Filip V., Plockova M., Smidrkal J., Cervenkova R. Inhibition of Aspergillus niger DMF 0801 by monoacrylglycerols prepared from coconut oil. Czech J Food Sci. 2002;20:48–52. [Google Scholar]
- 18.Schlievert P.M., Peterson M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE. 2012;7:1–12. doi: 10.1371/journal.pone.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seleem D., Chen E., Benso B., Pardi V., Murata R.M. In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms. PeerJ. 2016;4:1–17. doi: 10.7717/peerj.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishna S., Fujihara K., Teo W.E., Lim T.C., Ma Z. World Scientific Publishing; Singapore: 2005. An introduction to electrospinning and nanofibers. [Google Scholar]
- 21.Montgomery D.C. 7th ed. John Wiley & Sons; New York: 2009. Design and analysis of experiments. [Google Scholar]
- 22.Dooren A.A.V. Design for drug-excipient interaction studies. Drug Dev Ind Pharm. 1983;9:43–55. [Google Scholar]
- 23.Chen X., Wu D., He Y., Liu S. Detecting the quality of glycerol monolaurate: a method for using Fourier transform infrared spectroscopy with wavelet transform and modified uninformative variable elimination. Anal Chim Acta. 2009;638:16–22. doi: 10.1016/j.aca.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Limmatvapirat S., Panchapornpon D., Limmatvapirat C., Nunthanid J., Luangtana-Anan M., Puttipipatkhachorn S. Formation of shellac succinate having improved enteric film properties through dry media reaction. Eur J Pharm Biopharm. 2008;70:335–344. doi: 10.1016/j.ejpb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Mishra M.K. Solution properties of shellac, 4. Solubility parameter of shellac. Macromol Mater Eng. 1987;147:107–112. [Google Scholar]
- 26.Blaszyk M., Holley R.A. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int J Food Microbiol. 1998;39:175–183. doi: 10.1016/s0168-1605(97)00134-7. [DOI] [PubMed] [Google Scholar]
- 27.Dhekale N.H., Bindu K.H., Kirankumar K.Y., Gore A.N., Anbhule P.V., Kolekar G.B. Development and optimization of a multivariate RP-UPLC method for determination of telmisartan and its related substances by applying a two-level factorial design approach: application to quality control study. Anal Methods. 2014;6:5168–5182. [Google Scholar]
- 28.Candioti L.V., De Zan M.M., Camara M.S., Cámara M.S., Goicoechea H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta. 2014;124:123–138. doi: 10.1016/j.talanta.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Skrivanova E., Marounek M., Benda V., Brezina P. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet Med (Praha) 2006;51:81–88. [Google Scholar]
- 30.Bergsson G., Steingrimsson O., Thormar H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int J Antimicrob Agents. 2002;20:258–262. doi: 10.1016/s0924-8579(02)00205-4. [DOI] [PubMed] [Google Scholar]
- 31.Ouattara B., Simard R.E., Holley R.A., Piette G.J., Bégin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int J Food Microbiol. 1997;37:155–162. doi: 10.1016/s0168-1605(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 32.Limmatvapirat S., Limmatvapirat C., Luangtana-Anan M., et al. Modification of physicochemical and mechanical properties of shellac by partial hydrolysis. Int J Pharm. 2004;278:41–49. doi: 10.1016/j.ijpharm.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Schneider L.A., Korber A., Grabbe S., Dissemond J. Influence of pH on wound healing: a new perspective for wound therapy. Arch Dermatol Res. 2007;298:413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]