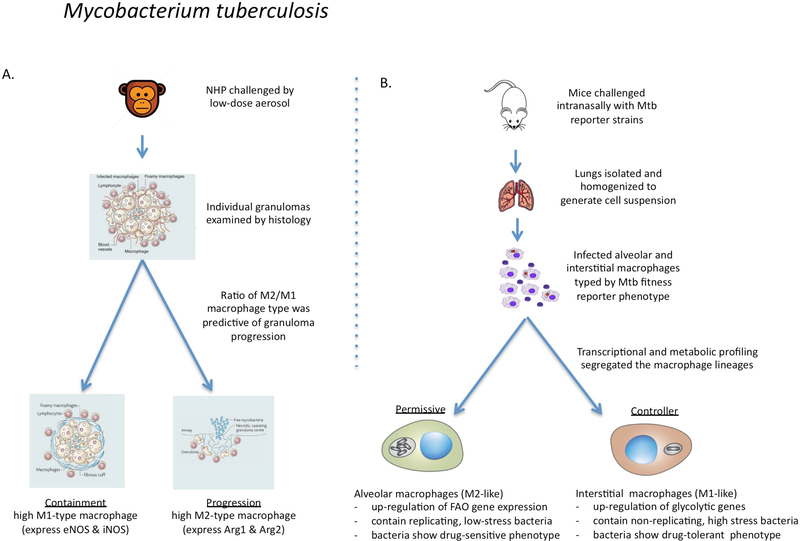
Figure 4 |. The links between macrophage phenotype and the progression of Mycobacterium tuberculosis infection in non-human primates and mice.
a | In macaque monkeys infected with Mycobacterium tuberculosis, the bacterial burden in individual granulomas and progression to active disease were found to correlate directly with an increased ratio of macrophages expressing M2 markers over Ml markers105,106. Progression was determined at the level of the individual granuloma without any consensus at the level of the host. b | In mice infected with fitness and replication reporter strains of M. tuberculosis, resident alveolar macrophages were more permissive of bacterial replication than were recruited interstitial macrophages107. RNA-sequencing showed that the alveolar macrophages were more committed to fatty acid oxidation (FAO) and oxidative phosphorylation, whereas the interstitial macrophages had higher levels of glycolysis. Selective depletion of the alveolar macrophage population reduced the bacterial burden, whereas depletion of the interstitial macrophage population led to an increase in bacterial burden. Therefore, in agreement with the data from non-human primates105,106, the ratio of alveolar (M2-like) macrophages to interstitial (M1-like) macrophages affects bacterial growth and disease progression in mice. In addition, drug-tolerant M. tuberculosis were more abundant in vivo in macrophages expressing M1-like activation markers95.