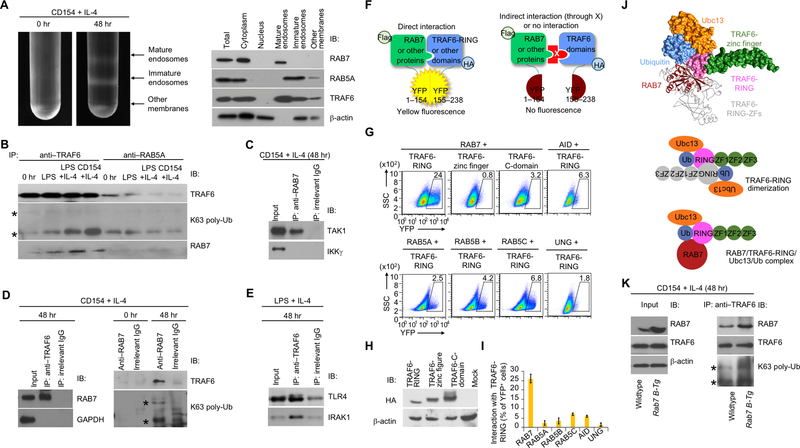
FIGURE 5.
RAB7 interacts with TRAF6 and enhances TRAF6 activity. (A) Intracellular membrane fractionation of B cells before or after CD154 plus IL-4 stimulation for 48 h (left) and detection of protein molecules in different member fractions by immunoblotting (right). (B) Immunoblotting analysis of proteins immunoprecipitated by anti-TRAF6 or anti-RAB5A IgG from B cells prior to stimulation (0 hr) or stimulated as indicated for 48 h (K63 poly-Ub, K63 polyubiquitination). Two bands denoted by “*” indicated K63 polyubiquitinated TRAF6. (C) Immunoblotting of proteins immunoprecipitated by anti-RAB7 IgG or by an irrelevant IgG from B cells stimulated with CD154 plus IL-4 for 48 h. (D) Immunoblotting analysis of proteins immunoprecipitated by anti-TRAF6 (left panels), anti-RAB7 (right panels) or an irrelevant IgG from B cells prior to stimulation (0 hr) or stimulated as indicated for 48 h. (E) Immunoblotting of proteins immunoprecipitated by anti-TRAF6 IgG or by an irrelevant IgG from B cells stimulated with LPS plus IL-4 for 48 h. (F) Illustration of the principle of the BiFC assay. (G) BiFC analysis of direct interaction of TRAF6-RING domain with RAB7, but not other proteins, as indicated. (H) Immunoblotting of expression of HA-tagged different TRAF6 domains. (I) BiFC analysis and quantified interaction of TRAF6-RING with different protein factors, as indicated (n=3, mean and s.e.m. in the histogram). (J) Immunoblotting analysis of total proteins (input) and proteins immunoprecipitated by anti-TRAF6 in stimulated B cells. (K) The interaction of RAB7 (red) with the TRAF6-RING domain (pink) in complex with ubiquitin (blue) and Ubc13 (orange), as modeled by docking analysis (top panel; PDB ID: RAB7 1yhn; TRAF6-RING/Ubc13/ubiquitin: 5vo0). In general, TRAF6 needs to be dimerized to catalyze K63 polyubiquitination, with the two RING domains in an anti-parallel conformation to stabilize the interaction with ubiquitin through reciprocal binding to the first zinc finger (ZF1, green; bottom panel). RAB7 can substitute the RING-domain and ZF1 of one TRAF6 monomer, as depicted by its overlaying of a TRAF6 monomer (grey, top panel), in stabilizing the interaction of TRAF6-RING and ubiquitin (middle panel). A-E, H, J, representative of two independent experiments.