Abstract
Complex regional pain syndrome (CRPS) is a condition of neuropathic pain, which is characterized by significant autonomic and inflammatory features. CRPS occurs in patients who have limb surgery, limb fractures, or trauma. Many patients may have pain resolve within twelve months of the inciting incident; however, a small subset progresses to the chronic form. This transitional process often happens by changing from warm CRPS with dominant inflammatory phase to cold CRPS, in which autonomic characteristics or manifestations dominate. Several peripheral and central mechanisms are involved, which might vary among individuals over a period of time. Other contributors include peripheral and central sensitization, autonomic alterations, inflammatory and immune changes, neurochemical changes, and psychological and genetic factors. Although effective management of the chronic CRPS form is often challenging, there are a few high quality randomized controlled trials that support the efficacy of the most commonly used therapeutic approaches.
Keywords: Neuroscience, Health sciences, Neurology, Surgery, Pain research, Pain management, CRPS, Pain, Pathophysiology, Treatment, Future therapy
Neuroscience; Health sciences; Neurology; Surgery; Pain research; Pain management, CRPS; Pain; Pathophysiology; Treatment; Future therapy.
1. Introduction
Complex regional pain syndrome (CRPS) is a form of spontaneous or stimulus-induced chronic pain that most often affects one limb (arm, leg, hand, foot) usually after an injury and lasting over six months [1]. Also, CRPS is previously known as Sudeck's atrophy (or dystrophy), algoneurodystrophy, algodystrophy, reflex neurovascular dystrophy, and reflex sympathetic dystrophy (RSD). CRPS is believed to be produced by dysfunction of the central and peripheral nervous systems [2]. CRPS is characterized by severe prolonged pain, changes in skin color and temperature, swelling, and bone loss in the affected limb [1, 2]. CRPS is divided into two types: I and II. Patients who have reflex sympathetic dystrophy syndrome without confirmed nerve injury are categorized as having CRPS-I [3].
However, CRPS-II, which is known as causalgia, occurs when there is associated and established nerve damage [3]. As there is no golden test for CRPS, there are many diagnostic criteria [4]. Also, the heterogeneity of patients' signs and symptoms makes it difficult to compare the studies to explain pathophysiological mechanisms or to evaluate treatment outcomes [5]. Consequently, this review aimed to reveal the updated therapeutic strategies based on the recent understanding of the pathophysiology of CRPS and to discuss novel approaches and techniques for managing this condition.
2. Research method
The databases used were PubMed, McGill University database, and the Cochrane database and MEDLINE, using the keywords “CRPS, pain, pathophysiology, treatment, and future therapy” and other old names or synonyms of CRPS before 1994, such as reflex sympathetic dystrophy syndrome, algodystrophy, and causalgia. The literature selected was focused on complex regional pain syndrome in patients who suffered from severe limb pain after surgery or trauma. However, reports that studied other kinds of pain were excluded, such as rheumatolic pain, visceral pain and psychogenic pain. Initially, we searched related published studies for the last three decades and identified studies that assessed the updated management and the pathophysiology of complex regional pain syndrome. The literature was reviewed and evaluated for quality and relevance, and our findings were summarized in this paper as following.
2.1. Study selection
Getting an adequate number of articles is the necessity for providing updated information, we recommend to select studies and accepted manuscripts based on abstracts and keywords for CRPS and were combined in searches of Web of Science for articles dated from 1989 to 2019. Then, the traditional snowball method was used to select literature that the clinical research team deemed most relevant. Also, inclusion and exclusion criteria were screened and selected by all of the authors. Inclusion criteria for extracting data were selected from the papers have written in the English language according to the abstract of systematic review and keywords. We exclude the articles have written in the non-English language.
2.2. Data extraction
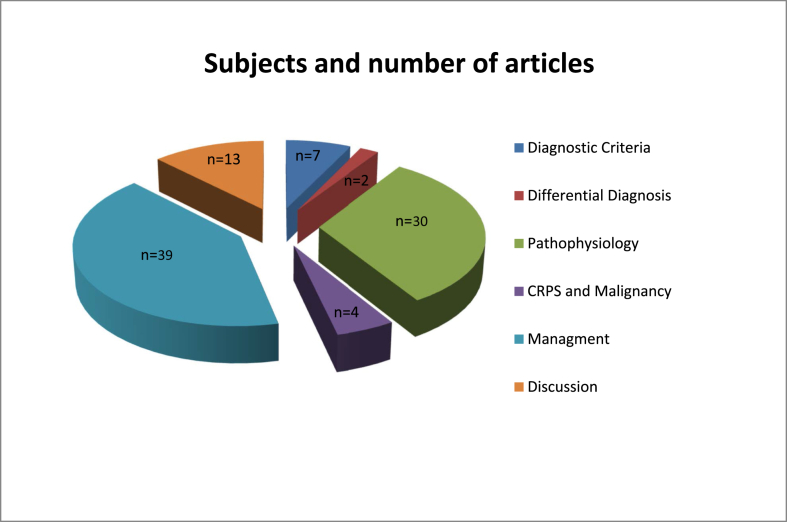
We retrieved 95 extracted papers by reviewing and analyzing data by Dr. Blaise, and also we are compared the data from the previously defined therapeutic interventions and findings in order to figure out the most frequent pathogenesis and potential novel therapeutic strategies for this condition. Therefore, obtained information was summarized and analyzed into specific parts to establish the research question (Figure 1).
Figure 1.
Research strategy: A graph representation.
2.3. Data analysis
There were no specific variables in these articles which have shown a strong evidence to compare and to analyze case reports, clinical studies and cross-sectional studies for establishing research question. The review has been organized at a high level heterogeneity, scalability, and consistency. Further, using theses measurements might have its strengths and weaknesses, thus the choice depends on the objective of the research and the availability of data.
3. Diagnostic criteria
Typically, there is no golden test for CRPS; therefore, the assessment of clinical criteria and an exclusion diagnosis are an entirely potential way to figure out the diagnosis of CRPS [5, 6]. Table 1. Illustrates the International Association for the Study of Pain (IASP) diagnostic criteria for CRPS.
Table 1.
IASP diagnostic criteria for CRPS [6].
CRPS I
|
However, these criteria were not widely accepted since they lack specificity and internal validity. Consequently, Bruehl and Harden have modified the IASP diagnostic criteria [2, 4, 6]. Whereas IASP criteria are based on patient-reported symptoms alone, the Harden/Bruehl criteria have necessitated physicians to assess and exam patient's signs in at least 2 out of 4 categories that are shown in Table 2 [6].
Table 2.
The Harden/Bruehl Criteria, which became The Budapest Research Criteria after the clinical decision rule is modified and adjusted to be at least 2 sign and at least 4 symptom categories [6].
|
Moreover, the Budapest consensus panel has suggested another subtype of CRPS, namely CRPS not otherwise specified (CRPS-NOS) which partially meets the CRPS diagnostic criteria and is not explained by other conditions [6]. Additionally, CRPS-NOS patients were diagnosed with CRPS, but recently, they do not meet the Budapest criteria [6]. However, much larger multiethnic and multinational patient samples are advised to be analyzed to eliminate the differences and increase the sensitivity of Budapest Research Criteria [6]. In contrast, in 1993, all patients had been presented at the outpatient clinic of the department of surgery, Nijmegen University Hospital, were clinically assessed the manifestations of RSD. As RSD was not clearly understood, thereby following specific criteria were used to assess patient's proportion, which meets the Veldman criteria, Table 3 [7].
Table 3.
The Veldman criteria [7].
|
In general, the key symptom of CRPS is prolonged intense pain that may be constant. It has been described as a “burning” or “pins and needles” sensation, or as if someone was squeezing the affected limb [3]. The pain might spread to the entire arm or leg, even though the injury might limited only to involve a finger or a toe. In rare cases, pain can sometimes travel to the opposite extremity. The affected area has often increased the sensitivity, known as allodynia, in which regular contact with the skin is experienced as very painful [3, 8].
Based on the previous studies, which have concluded patients with CRPS have experience changes in skin temperature and color and swelling of the affected area [4]. This condition occurs due to abnormal microcirculation caused by damage to the nerves controlling blood flow and temperature [3, 4, 8]. As a result, an affected limb may feel warmer or cooler compared to the opposite limb. In addition, the skin on the affected area might have changed color, becoming blotchy, blue, purple, or pale [3, 4, 5, 8].
Common manifestations of CRPS
-
•
Stiffness in affected joints.
-
•
Change in nail and hair growth patterns.
-
•
Change in skin texture on the damaged area; it may appear shiny and thin.
-
•
An abnormal sweating pattern in the affected and surrounding areas.
-
•
Coordinating muscle movement disorder, with decreased ability to move the affected body part.
-
•
Abnormal movement in the involved limb, most often fixed abnormal posture (dystonia) with jerking of the affected part [4, 5].
3.1. Differential diagnosis
CRPS diagnosis is mainly based on medical history and clinical examination with different tests that can help to exclude other diagnoses, Table 4 [9]. Indeed, the neurological examination is extremely significant, such as conduction velocity studies to exclude nerve lesions [9]. However, electromyography procedures could be unnecessary because they are so painful, and their results do not have any effect on the therapy [9]. Also, quantitative sensory testing (QST) or bedside sensory testing is helpful to detect small fiber dysfunction [9]. In order to assess central pathways, the previous producers should be accompanied with transcranial magnetic stimulation of motor pathways and somatosensory evoked potential studies [9]. Additionally, clinicians and pain specialties should be aware to exclude the other medical conditions by ordering investigations, such as severe skin infections and chronic rheumatic diseases as summarized in Table 4 [10].
Table 4.
Technical investigations used to exclude differential diagnosis [10].
| Investigation/parameter | Exclusion/suspicion of |
|---|---|
|
Infection (e.g., postoperative) |
|
Rheumatic disease |
|
|
|
Fracture, nonfusion, osteoarthritis, |
| Rheumatic arthritis, osteomyelitis, | |
|
Rheumatic arthritis, polyarthritis, |
| Osteoarthritis, Osteomyelitis | |
|
Fatigue, fracture, nonfusion, |
| Osteomyelitis, tendovaginatis |
4. Pathophysiology
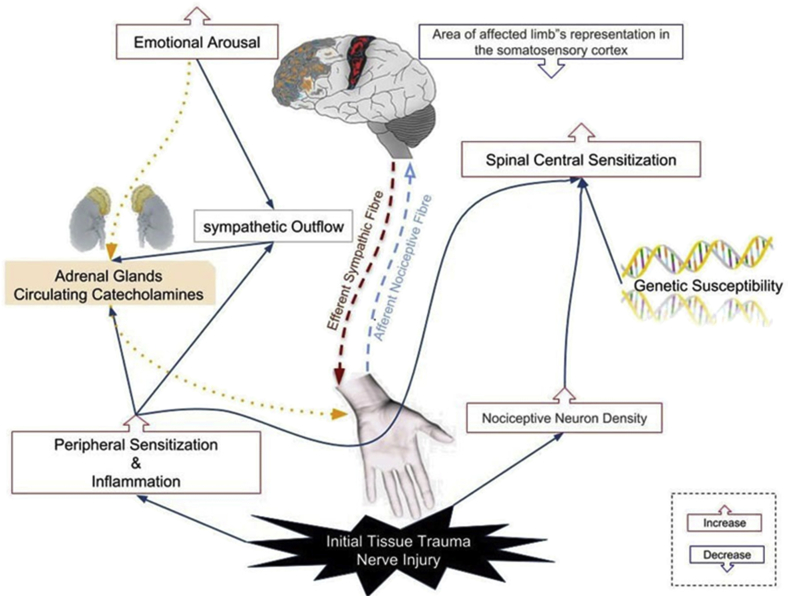
There is a recent universal agreement that CRPS is caused by a multifactorial process that involves both the peripheral and central nervous systems [11]. There is evidence of each of the mechanisms that have a role in the development of CRPS, although there is little experimental data about how these mechanisms may have interacted to produce this syndrome [12]. The variety of manifestations seen in CRPS depends on the relative contributions of different processes that can differ among patients over time (Figure 2) [5, 6, 12]. For instance, fracture or sprain causes have represented approximately 60% of CRPS patients, and the symptoms are extensive wide and severe. Many clinical trials have been categorized the remaining alleged events that comprise the other estimated 40% are even more imprecise, and in some CRPS cases, causes are not recognized [12]. Therefore, we will discuss the pathophysiological mechanisms that are involved in the development of CRPS.
Figure 2.
The speculative model illustrates the interacting different pathophysiological mechanisms of complex regional pain syndrome.
4.1. Inflammatory mechanism
CRPS patients supposedly exhibit all the signs of inflammation, such as heat, pain, redness, and swelling [6]. Several clinical trials, CRPS symptoms have been significantly improved by corticosteroids, suggesting that inflammatory mechanisms contribute to CRPS, especially in the acute phase [13]. Some studies have detected the presence of Langerhans cells in skin biopsies from CRPS patients and another study has observed cellular infiltration, mainly lymphocytes, in synovial biopsy specimens [6,14]. Also, the inflammatory process that contributes to CRPS might result from two inflammatory cascades. Firstly, the classic inflammatory mechanisms through immune cells actions, such as mast cells and lymphocytes [15]. Post-traumatic soft tissue, mast cells and lymphocytes release proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin-1β, -2, and 6. Subsequently, these substances elevate plasma extravasation and cause localized edema [15].
Secondly, neurogenic inflammation mechanisms through the direct release of neuropeptides and proinflammatory cytokines from nociceptive fibers contribute CRPS in response to different triggers, e.g. nerve injury [16]. Neuropeptide mediators (calcitonin gene-related peptide (CGRP), substance P, and bradykinin) increase plasma extravasation and cause vasodilation, leading to the warm, red, and edematous [16]. In addition, the concept of neurogenic inflammation includes that distinct classes of C-fibers have an afferent mediation function of pain and itch and an efferent neuro-secretory function [17]. Significantly, mechano-heat-insensitive C-fibers (C-MiHi), which is known as silent nociceptors because they don not response to a physiological or mechanical stimulus. These chemoreceptors and released neuropeptides are stimulated via inflammatory mediators [18,19], which lead to activate central sensitization (e.g. the secondary mechanical hyperalgesia development) by C-MiHi [20]. Several studies have proven the increase in neuropeptides release in CRPS patients and normalize the releasing level after sufficient therapy [17,20,21]
Additionally, CGRP increases oedema, vasodilatation, and sweating by a peripheral mechanism (the symptoms that are well observed in CRPS patients), and neuropeptides and proinflammatory cytokines cause peripheral sensitization that lead to stimulate nociceptive responsiveness [[16], [22]]. Many research have been carried out on patients with CRPS in order to prove a significant increase in proinflammatory cytokines [23], and a reduction in anti-inflammatory cytokines systemic levels (interleukin-10) that might exaggerate the inflammatory mechanism [24]. Also, elevated CGRP and neuropeptides levels may cause trophic and autonomic signs, such as swelling, hyperhidrosis, and erythema [15]. Thus, inflammatory factors are considered as the cardinal features of CRPS.
4.2. Altered cutaneous innervation
Even though CRPS-I is a dystrophy syndrome without confirmed clinical nerve injury, a recent study believed that has shown an initial nerve trauma is a significant trigger for CRPS cascade [1,10]. This hypothesis is approved by skin biopsy examination, which was obtained from CRPS-I patients who did not have any clinical signs of nerve injury [25]. In a clinical study, the densities of epidermal neurites were up to 29% lower in CRPS-affected limbs compared to unaffected limbs; thus these changes might affect nociceptive fibers [25]. Even though C-fibers are stimulated in CRPS, a clinical study has shown that a significant loss in C-fiber and Aδ-fiber density was reported in CRPS-I affected limbs [26]. Moreover, innervation alteration around sweat glands and hair follicles was detected in CRPS-I patients. In vivo study has highlighted that the change in distal extremity innervation in CRPS-I might be an outcome of the injury triggering CRPS. However, more clinical trials are requested to prove the relation between neurite loss and injury initiating CRPS [26,27].
4.3. The sympathetic nervous system
The clinical course of CRPS during its chronic phase is characterized by the affected area being bluish and cold due to vasoconstriction. This mechanism has suggested by excessive sympathetic nervous system function activity, which is considered an indicator of disease progression and contributing factor for the pain [28]. Experimental studies have reported that expression of adrenergic receptors on nociceptive fibers after tissue damage and nerve trauma might provide a potential mechanism for sympathetically induced pain [11,29].
4.4. Role of circulating catecholamines
The clinical presentation of CRPS depends on various processes that progress from the acute phase to the chronic phase, and could lead to attribute to change in catecholaminergic actions. Therefore, during the acute phase, the injured part has reduced levels of circulating plasma norepinephrine compared to an unaffected area [11,30]. Also, there is a compensatory upregulation mechanism at peripheral adrenergic receptors that may lead to higher sensitivity to circulating catecholamines [30].
4.5. Autoimmunity
It has been suggested that autoimmunity might play a role in the development of CRPS by the presence of immunoglobulin G (IgG) autoantibodies surface-binding to autonomic neurons in the serum of CRPS patients [31,32]. This would be supported by the findings of a small pilot study where patients with CRPS were given intravenous immunoglobulin treatment. The therapy demonstrated a significant reduction in pain symptoms when compared with placebo [33].
4.6. Brain plasticity
Neuroimaging testing of CRPS patients has observed a significant decrease in a region of the somatosensory cortex that represents the CRPS-affected body part compared to that of the unaffected area [34, 35, 36]. Therefore, this extension of sensory damage may be significantly correlated with the pain intensity and degree of hyperalgesia experienced in patients with CRPS. These alterations would return to normal after successful CRPS management [37].
4.7. Genetic effects
However, there is a lack of compelling evidence regarding the effect of genetic factors on CRPS; some family-based studies have reported a genetic predominance for developing this condition [38]. Further, some genes of the major histocompatibility complex are related to this condition, such as human leukocyte antigen (HLA) molecules, HLA-B62, and HLA-DQ8 allele were found to be highly correlated as potential pathogenesis for CRPS [39].
4.8. Psychological influence
Chronic persistent pain affects the health-related quality of life and the emotional and psychological wellbeing of patients with CRPS [40]. The prevalence of psychological disorders in patients with CRPS, such as anxiety and depression, and the unusual nature of clinical manifestations were suggested to play a role in the development of CRPS. After fractures of the distal radius in elderly patients, these psychological factors were observed at a higher rate of occurrence in CRPS [40]. However, there is no clear evidence to confirm this correlation, and other studies have failed to prove this relation [11,40].
4.9. CRPS and malignancy
Several studies have revealed that complex regional pain syndrome type I is unusually associated with a variety of malignancies, which might lead to intense pain in debilitating patients [41]. The relationship between cancer and CRPS was observed since long time ago nearly 60 years. The majority of these patients had diagnosed in upper limbs CRPS-I within a limited periods from 4 to 6 months were recorded from the onset of CRPS-I to actual diagnosis of malignancy [41].
Occult cancer should be suspected and considered when CRPS type I presents itself in the absence of a clear explanation, particularly in cases with a high risk of developing cancer. There are numerous studies have proven that CRPS-I may have involving gynecologic cancers, such as cervical and vulvar malignancies that may lead to CRPS-I in the lower extremities. However, ovarian tumors can commonly cause upper extremity CRPS-I [41]. More recently, case report has found out the metastasized lung cancer adenocarcinoma to left proximal phalanx of the third digit that leads to diagnose complex regional pain syndrome, as a result there was no clinical improvement of that lesion over a period of 3 months before presenting to the emergency department [42].
In order to diagnosis of CRPS-I in patients associated with malignancies is usually depended on clinical features after excluding other existent diseases, which could be accounted by assessing the intensity of pain and dysfunction [41]. Furthermore, the diagnostic criteria of CRPS-I may increase by requiring at least two positive criteria and four categories of clinical manifestations that may help to differentiate CRPS-I in cancer patients [41,43].
5. Management
Based on reports, some CRPS cases can be resolved with conventional medical therapies, but in general, the management of CRPS may be a difficult challenge for clinicians and pain specialties as the syndrome is a complex physio-psychosocial condition [44]. Thus, chronic CRPS is suggested to be treated with a comprehensive, multidisciplinary approach that includes medical, psychological, and physical and occupational therapy components [45]. However, many clinical trials in CRPS have been increasing in recent years, the therapeutic approaches used and the results obtained are the subjects of much controversy [46].
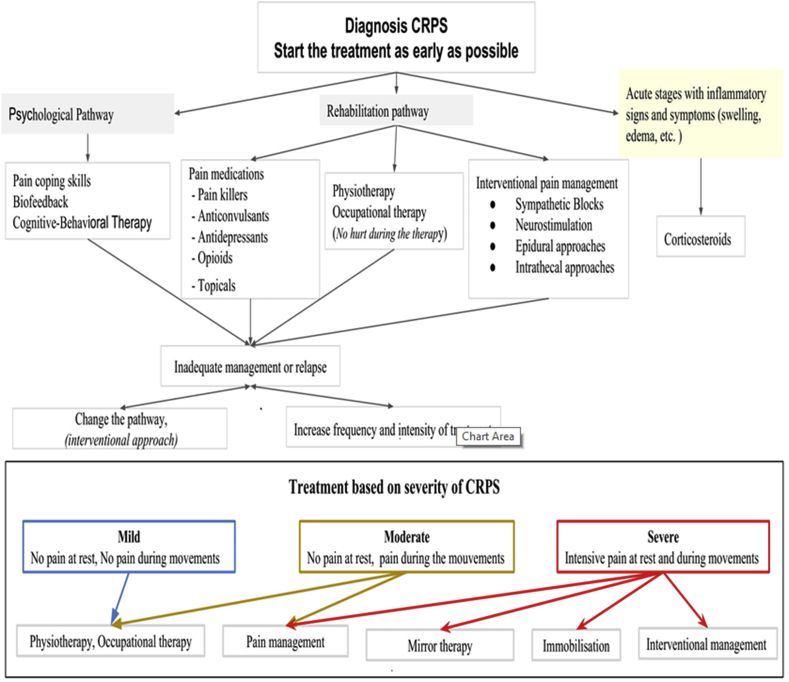
This review aims to examine the benefits from physical, occupational and psychological therapies (Figure 3), as well as to find out the evidence for the safety and efficacy of pharmacological interventions, such as sub-anesthetic intravenous ketamine, bisphosphonates, surgical sympathectomy, spinal cord stimulation and other new therapeutic strategies [46,47].
Figure 3.
The Summarized algorithm of management CRPS that explains multiple therapeutic approaches and interventions depend on the severity of the condition.
5.1. Physical and occupational therapies
Physical and occupational therapies are important steps in the rehabilitation process in patients with CRPS. Patients might have kinesophobia (the pathological fear of motion), and the goal of treatment is to overcome fear of pain. This approach could involve several therapeutic modalities [46,48]. These include massage, elevation, and transcutaneous electrical nerve stimulation, and contrast baths, gentle range of movement of the affected limb, strengthening exercises, and stress loading of the involved part along with providing of adequate analgesia. As a result, this kind of therapy encourages CRPS patients to use the affected limb in daily activities [49,50].
5.2. Pharmacological interventions
5.2.1. Anti-inflammatory medications
Although the efficacy of NSAIDs in reducing neuropathic pain in some medical conditions has not been proved yet, the inflammation process has a role in CRPS pathogenesis, especially in the early stages [51]. Thus, these medications work by suppressing cyclooxygenase-1 and -2, leading to a decrease in prostaglandins secretions that exaggerate inflammatory mechanism. This could lead to inhibit an overall anti-inflammatory process and reduce pain severity. Several studies have been involved the prescribing of NSAIDs in CRPS were not sufficient, and results have been controversial [52,53]. In 2014, a group of individuals had been examined for the short term use of parecoxib to control the intensity of pain and swelling in CRPS patients [49]. The study sample was twenty CRPS patients with upper limb injured were recruited and randomised for receiving intravenous parecoxib 80 mg for 2 days or placebo each day [49]. The findings have shown no differences between the two groups [52].
Additionally, there is a study which has compared the efficacy of the corticosteroid vs the NSAIDs in patients with CRPS type I after the cerebrovascular attack; thus the results have shown that the group taking steroid had been had significantly improved in signs and symptoms of CRPS in compared to the group taking NSAIDs [54]. In 2010, a randomised study had investigated whether a single dose of intrathecal methylprednisolone 60 mg in patients with chronic CRPS might have a positive effect on controlling pain severity and reducing inflammation in involved limb [54]. However, administrating steroid for a long-term period and its adverse effects have been well established [54].
5.2.2. Bisphosphonates
Bisphosphonates have been suggested to use for CRPS management; however, the mechanism of pain reduction is not well understood [55]. Some theatrical research has been involved in the ability of bisphosphonates for modulating inflammatory mechanisms, suppressing bone marrow cell growth, and decreasing the bone microenvironment acidity [54,56]. In 2013, a Cochrane article has been published, which is indicated the evidence for use bisphosphonates as an effective for pain intensity in patients with CRPS was of low quality and insufficient. Based on the most recent meta-analysis, the investigators concluded that bisphosphonates have therapeutic efficacy for reducing pain in a patient with CRPS; however, further research was needed [44].
5.2.3. Ketamine infusion
Using Ketamine to manage CRPS patients is based on its action to block NMDA receptors. Experimental evidence has suggested that the clinical manifestations of CRPS produced by a sufficient painful stimulus that could lead to increase and extend glutamate release from first order nociceptive afferents [57]. Also, the releasing glutamate stimulates NMDA receptors on second-order neurons within the spinal cord that lead to central sensitization. Therefore, blocking NMDA receptors might also prevent cellular supporting mechanisms. Further, several studies were shown successful management of patients had been considered refractory to traditional CRPS treatments with low-dose ketamine infusions [57].
A randomized placebo-controlled trial revealed that IV ketamine with the rage of dose 0.35 mg/kg over 4 h for ten days found out a significant pain reduction in patients with CRPS. Additionally, a study has reported potent analgesia of transient duration while other studies revealed with more prolonged infusions for two weeks, as well as have shown analgesia extended up to 3 months [58,59].
5.2.4. Neuropathic pain medications
Neuropathic pain medications have not been potentially investigated for treating CRPS. Based on their safety and efficacy in treating other neuropathic diseases; therefore, the evidence of using neuropathic pain medications for managing patients with CRPS is limited [60]. For example, Gabapentin which works by binding to the a2-d subunit of voltage-dependent calcium channels, has proven the efficacy in pain control in patients with CRPS [61,62]. More recently, in 2016, a clinical trial has investigated amitriptyline vs gabapentin for CRPS -I in the pediatric age group was found both medications were effective in improving sleep and minimizing pain severity, as well as there is no statistically significant difference between the medications [63]. Thus, the administration of other neuropathic pain agents by pain specialists for treating patients CRPS is empirical and depends on each clinician's preference and experience [63].
5.2.5. Intravenous immunoglobulin
A clinical study has found that intravenous immunoglobulin (IVIG) can reduce the pain intensity in patients suffering from CRPS [64]. IVIG has been suggested as a novel therapeutic modality for chronic CRPS patients. The best evidence has been figured out from a double-blind, placebo-controlled cross-over trial in 13 patients who received IVIG 0.5 g/kg or saline, were separated by a washout period of greater than 28 days. The results illustrated by the average pain severity that was reduced by 1.55 on a 0–10 pain rating scale in the IVIG group [33,65]. We need more studies in future for supporting IVIG has a role in treating patients with CRPS.
5.2.6. Intravenous magnesium
The efficacy of IV magnesium in CRPS is very controversial [66]. Although some studies showed it to be a good choice for acute stage CRPS type I management, others showed magnesium have no significant benefit over placebo [66].
5.2.7. Epidural clonidine
Double-blind and controlled trials have resulted in a statistical significance of pain control from epidural clonidine injections in patients with CRPS. However, they also revealed significant adverse effects with both single injections and continuous epidural infusions [67]. A clinical study has shown the difference of epidural infusion of clonidine with range 300 μg and 700 μg, or normal saline in random order [67]. The researchers investigated the findings by comparing to the placebo group; therefore, the pain was notably reduced in both treatment groups throughout the study period [68] with appreciate and monitor the adverse events of clonidine (e.g. hypotension).
5.2.8. Novel compound analgesic cream (CAC)
A study has reported evidence of topical combinations of α-2 adrenergic receptor agonists or nitric oxide (NO) donors with phosphatidic acid inhibitors. These agents formulated to manage microvascular damage and to decrease allodynia in a rat model of CRPS type I [69]. Based on these findings, researchers assessed the outcomes of CRPS patients who were treated with a compound analgesic cream (CAC) that consisted of 10% of ketamine, 6% of pentoxifylline, 0.2% clonidine, and 6–10% of dimethyl sulfoxide [69]. The results have significantly supported the topical combination of ketamine, pentoxifylline, and clonidine as a potential therapeutic regimen for patients with CRPS. Over 50% of patients reported benefits with the use of CAC and pain reduction. Further, the CAC has potentially demonstrated to resolve CRPS symptoms and carried out the resolution of a pre-CRPS diagnosis, as well as the importance of recognizing this progressive syndrome in its early stages [69].
5.3. Surgical interventions
Neuromodulation might have a role in managing in patients with CRPS, especially those who are unresponsive to sympathetic blockade procedure [70]. One RCT has been found out that spinal cord stimulation (SCS) and physiotherapy are effective for reducing pain in compared to physiotherapy alone at 6 months and 2 years although this effectiveness decreased at a long-term follow up of 5 years [71].
5.3.1. Spinal cord stimulation
If pharmacotherapy has failed to improve a patient's condition, it is very crucial to move on to a trial of spinal cord stimulation (SCS) [72]. It has been shown that two-thirds of patients treated with permanent SCS implantations had improved function and normalizing daily activities [73]. Therefore, there would be significant evidence to support the value of SCS in the management of patients with CRPS, who have experienced lower benefit from traditional therapies [73].
5.4. Plasma exchange
More recently, understanding of the autoimmune etiology of CRPS has indicted the essential use of plasma exchange treatment, which has demonstrated benefit in other autoimmune disorders [74]. A clinical study, 91% of patients stated significant pain relief of 64% following treatment. Also, weekly therapy was shown to be successful in sustained pain control in 45% of patients with CRPS [74].
5.5. Others
Topical preparations of antioxidants such as dimethyl sulfoxide (DMSO) and N-acetylcysteine have been potentially revealed decreasing pain intensity [72]. More recently, vitamin C has proven as the most effective preventative therapy for developing CRPS and is commonly administrated following extremity surgery [75,76]. Also, mannitol (a free radical scavenger) has been reported to attenuate the inflammatory cascades induced by oxygen due to tissue damage in CRPS. However, these results are controversial with other studies [77,78].
5.6. Future therapy
Our aim in this review is to continue and to grow understanding the CRPS pathophysiology, as well as our scope for the finding out a novel or existing agents to manage the various disease mechanism cascades. The neurogenic inflammation that occurs in CRPS could be attributed in part to the activation of microglia, which is regulated by the toll-like receptor (TLR) signaling. For example, naltrexone has antagonistic effects at the TLR-4 and, hypothetically, suppresses inflammatory mechanism [79, 80, 81]. Therefore, administrating low dose of naltrexone increases opioids activity and significantly reduced pain in CRPS patients [81,82]. Other therapeutic approaches for CRPS are based on the vascular effect on endothelial cell production and function that act by increasing nitric oxide synthase, and rising carbon monoxide level, as well as decreasing free radicals production in endothelial cells.
6. Discussion
CRPS is mistakenly referred to as chronic regional pain syndrome. Chronic regional pain syndrome has been named many various synonymous terms, including Sudek's atrophy, reflex sympathetic dystrophy, shoulder-hand syndrome, and algodystrophy [83]. The term CRPS has been used after being reviewed and renamed for several years; evidence that many aspects of the disease are unclear [83]. The confusion part is due to the variety of applied diagnostic criteria for CRPS. Clinicians experience and clinical standards are suggested the use of the Budapest criteria for diagnosis and to clarify the clinical presentation of CRPS [83] because the pathophysiological process for CRPS is not well understood [83].
Early theories had just focused on hyperactive sympathetic responses causing the manifestations of CRPS [84]. Additionally, the decreased adrenergic outflow from sympathetic neurons is compensated by latent receptor up-regulation while increasing receptor capacity, synaptic potency, and exaggerated response to natural neurotransmitter might also lead to provoking symptoms in patients with CRPS [85].
In the absence of a standard golden test for the diagnosis of CRPS may lead to over or under diagnosis of this condition [86]. CRPS can be defined as a complicated condition for both patients and clinicians. Recently, according to the European Pain Federation task force provide 17 standards of the diagnosis and treatment of CPRS in Europe [86]. These are considered achievable for most countries, and aspirational for a minority of countries depending on their healthcare resources and structures. Therefore, the clinicians should quickly help to narrow down the differential diagnoses [87]. For instance, inflammatory arthropathies, which affect the hand and wrist, including rheumatoid arthritis, and gout, as well as infection, should be involved as a differential diagnosis. Some of the radiographic features of CRPS show a normal joint space and margin. This would help in differentiating CRPS from certain infectious conditions or some rheumatic conditions. Although the clinical findings of CRPS and gout do have several similarities, the radiographic findings for both gout and psoriatic arthritis are not consistent with those of CRPS [87,88].
Para-clinical testing is essential for providing information about the sensory, motor and autonomic changes [89,90]. In order to evaluate vascular and bone changes, we could use bone scintigraphy by providing simple radiography that is performed only in the chronic phase when the condition of the mineralization could be evaluated. Therapeutically, sympathetic blockade procedures were used as a diagnostic test and the management of chronic CRPS; however, some studies have shown that there is insufficient evidence reported pain and motor function improvement after sympathetic blockades. While many patients with CRPS have reported a positive effect after the sympathetic blockade, in contrast, others stated little success [87,89,90].
Recently, there has been a shift towards re-instituted functional abilities as the primary goal of treatment rather than pain control. Therapeutic exercise is one of the critical elements of this approach. Physiotherapy is an essential treatment, although there is controversy whether or not the severe activity can be harmful at an early stage [87]. A purpose of therapy is to improve joint movement, and passive strength movements are often unwanted and so painful and must be avoided in the acute phase [87,89]. Passive treatment, followed by isometric and isotonic physiotherapy, can be done when the level of pain decreases. Therefore, a multidisciplinary approach for treating CRPS patients should be considered, and the primary purpose of pharmacological interventions is to eliminate pain [88, 89, 90].
In the beginning, under the premise that neurologic inflammation is a pathologic mechanism, NSAIDs and steroids can be prescribed as a type of pain control agents. There is no long-term study of the use of these medications in the treatment of CRPS or other neuropathic pain; however, this is an initial treatment for pain control [91]. Also, tricyclic antidepressant drugs have been shown to have analgesic effects when used at lower doses than those required to produce antidepressant effects. The effectiveness of TCA in CRPS patients may be related to its interrupting the cascade of pain by sleep and mood improvement [91,92].
Additionally, for evidence-based practitioners, the practice of chiropractic has evolved to become more than merely joint manipulation. Recent studies suggest an increased awareness that many conditions require particular components of physiotherapy based interventions, including specific exercise regimens, as well as the recent two therapies include mirror therapy and graded motor imagery [54,93]. Few studies have been published on the chiropractic treatment of CRPS and similar conditions, although a case report on the chiropractic treatment of CRPS demonstrated an improvement in upper limb symptoms [90]. Because of the different presentation of symptoms and the extent of the condition, it can be challenging to determine which conservative therapies would be most beneficial in the treatment of CRPS. Clinicians and pain specialties must establish precise diagnoses and treatment protocols for this group of patients; more research is needed on therapies, such as chiropractic care, acupuncture, nutrition, massage and exercises related to physiopathology [94,95].
7. Conclusion
Although complex regional pain syndrome is an uncommon condition in the general population, it might happen in individuals who have a crush injury or post limb surgery. Several mechanisms of CRPS may be evident, both peripherally and centrally-involved, and these might differ across patients and even within patients over time. Recent clinical trials suggested that the most commonly used therapeutic interventions (sympathetic block) are probably ineffective for average patients. Also, the evidence-base regarding CRPS type II remains scarce as compared to CRPS type I. While there is still no successful therapy for CRPS to date, years of research have provided us with much valuable information and understanding of this condition's mechanisms. Continued effort is needed to explain the subgroups of patients who would benefit from the most currently available management. It is quite challenging to target a specific mechanism; however, a multidisciplinary approach is recommended for the management of CRPS patients. Therefore, the future of CRPS treatment may depend on combination therapy in terms of medical and surgical interventions, as well as studies investigating these approaches are necessary.
8. Summary
-
•
CRPS is clinically diagnosed.
-
•
No definitive diagnostic test is considered to be a gold standard for CRPS.
-
•
The pathophysiology of CRPS is still poorly understood. Peripheral and central sensitization and neuroimmune mechanisms are thought to play essential roles in CRPS.
-
•
CRPS might appear as a complication of various malignancies in advanced stages.
-
•
The management of CRPS depends on a multidisciplinary team approach, such as pharmacologic, interventional and neuromodulation.
-
•
CRPS is continuously developing and evidence-based managements for favorable results are insufficient.
-
•
Chronic pain and limb disability associated with CRPS may lead to psychological stress and depression.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Marinus J., Moseley G.L., Birklein F., Baron R., Maihöfner C., Kingery W.S., van Hilten J.J. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10(7):637–648. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruehl S., Harden R.N., Galer B.S., Saltz S., Bertram M., Backonja M., Stanton-Hicks M. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. Pain. 1999;81(1-2):147–154. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Neurological Disorders and Stroke . Department of Health and Human Services; Bethesda, Maryland: 2013. Complex Regional Pain Syndrome. 20892-2540. [Google Scholar]
- 4.Van de Beek W.J., Schwartzman R.J., Van Nes S.I., Delhaas E.M., Van Hilten J.J. Diagnostic criteria used in studies of reflex sympathetic dystrophy. Neurology. 2002;58(4):522–526. doi: 10.1212/wnl.58.4.522. [DOI] [PubMed] [Google Scholar]
- 5.Harden R.N., Bruehl S., Stanton-Hicks M., Wilson P.R. Proposed newdiagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8(4):326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 6.Borchers A.T., Gershwin M.E. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun. Rev. 2014;13(3):242–265. doi: 10.1016/j.autrev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Veldman P.H., Reynen H.M., Arntz I.E., Goris R.J. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012–1016. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- 8.Stanton-Hicks M. Fundamentals of Pain Medicine. Springer; Cham: 2018. Complex regional pain syndrome; pp. 211–220. [Google Scholar]
- 9.Harden R.N., Oaklander A.L., Burton A.W., Perez R.S., Richardson K., Swan M., Barthel J., Costa B., Graciosa J.R., Bruehl S. Complex regional pain syndrome: practical diagnostic and treatment guidelines. Pain Med. 2013;14(2):180–229. doi: 10.1111/pme.12033. 1. [DOI] [PubMed] [Google Scholar]
- 10.Birklein F., Schlereth T. Vol. 156. International Association for the Study of Pain; 2015. Complex regional pain syndrome—significant progress in understanding. Pain; pp. S94–103. [DOI] [PubMed] [Google Scholar]
- 11.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113(3):713–725. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- 12.Chang C., McDonnell P., Gershwin M.E. Complex regional pain syndrome–False hopes and miscommunications. Autoimmun. Rev. 2019 doi: 10.1016/j.autrev.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Calder J.S., Holten I., McAllister R.M. Evidence for immune system involvement in reflex sympathetic dystrophy. J. Hand Surg. 1998;(2):147–150. doi: 10.1016/s0266-7681(98)80162-9. [DOI] [PubMed] [Google Scholar]
- 14.Braus D.F., Krauss J.K., Strobel J. The shoulder–hand syndrome after stroke: a prospective clinical trial. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1994;36(5):728–733. doi: 10.1002/ana.410360507. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J.K., Ji R.R. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem. Res. 2008;33(10):1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birklein F., Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci. Lett. 2008;437(3):199–202. doi: 10.1016/j.neulet.2008.03.081. [DOI] [PubMed] [Google Scholar]
- 17.Herbert M.K., Holzer P. Neurogenic inflammation. I. Basic mechanisms, physiology and pharmacology. Anasthesiol. Intensivmed. Notfallmed. Schmerztherapie: AINS. 2002;37(6):314–325. doi: 10.1055/s-2002-32233. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt R., Schmelz M., Forster C., Ringkamp M., Torebjork E., Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J. Neurosci. 1995;15(1):333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmelz M., Michael K., Weidner C., Schmidt R., Handwerker H.O. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport. 2002;11(3):645–648. doi: 10.1097/00001756-200002280-00041. 28. [DOI] [PubMed] [Google Scholar]
- 20.Klede M., Handwerker H.O., Schmelz M. Central origin of secondary mechanical hyperalgesia. J. Neurophysiol. 2003;90(1):353–359. doi: 10.1152/jn.01136.2002. [DOI] [PubMed] [Google Scholar]
- 21.Weber M., Birklein F., Neundörfer B., Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91(3):251–257. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 22.Leis S., Weber M., Schmelz M., Birklein F. Facilitated neurogenic inflammation in unaffected limbs of patients with complex regional pain syndrome. Neurosci. Lett. 2004;359(3):163–166. doi: 10.1016/j.neulet.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Alexander G.M., Van Rijn M.A., Van Hilten J.J., Perreault M.J., Schwartzman R.J. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116(3):213–219. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Üçeyler N., Eberle T., Rolke R., Birklein F., Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain. 2007;132(1-2):195–205. doi: 10.1016/j.pain.2007.07.031. 1. [DOI] [PubMed] [Google Scholar]
- 25.Oaklander A.L., Rissmiller J.G., Gelman L.B., Zheng L., Chang Y., Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006;120(3):235–243. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht P.J., Hines S., Eisenberg E., Pud D., Finlay D.R., Connolly M.K. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120(3):244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Siegel S.M., Lee J.W., Oaklander A.L. Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth. Analg. 2007;105(6):1820–1829. doi: 10.1213/01.ane.0000295234.21892.bc. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen L.F., Terkelsen A.J., Drummond P.D., Birklein F. Complex regional pain syndrome: a focus on the autonomic nervous system. Clin. Auton. Res. 2019:1–11. doi: 10.1007/s10286-019-00612-0. [DOI] [PubMed] [Google Scholar]
- 29.Baron R., Schattschneider J., Binder A., Siebrecht D., Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case–control study. Lancet. 2002;359(9318):1655–1660. doi: 10.1016/S0140-6736(02)08589-6. [DOI] [PubMed] [Google Scholar]
- 30.Kurvers H., Daemen M., Slaaf D., Stassen F., van den Wildenberg F., Kitslaar P. Partial peripheral neuropathy and denervation induced adrenoceptorsupersensitivity. Functional studies in an experimental model. Acta Orthop. Belg. 1998;64(1):64–70. [PubMed] [Google Scholar]
- 31.Kohr D., Tschernatsch M., Schmitz K., Singh P., Kaps M., Schafer K.H. Autoantibodies in complex regional pain syndrome bind to a differentiation-dependent neuronal surface autoantigen. Pain. 2009;143(3):246–251. doi: 10.1016/j.pain.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Dubuis E., Thompson V., Leite M.I., Blaes F., Maihofner C., Greensmith D. Longstanding complex regional pain syndrome is associated with activating autoantibodies against alpha-1a adrenoceptors. Pain. 2014;155(11):2408–2417. doi: 10.1016/j.pain.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Goebel A., Baranowski A., Maurer K., Ghiai A., McCabe C., Ambler G. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann. Intern. Med. 2010;152(3):152–158. doi: 10.7326/0003-4819-152-3-201002020-00006. [DOI] [PubMed] [Google Scholar]
- 34.Maihofner C., Handwerker H.O., Neundorfer B., Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707–1715. doi: 10.1212/01.wnl.0000098939.02752.8e. [DOI] [PubMed] [Google Scholar]
- 35.Cappello Z.J., Kasdan M.L., Louis D.S. Meta-analysis of imaging techniques for the diagnosis of complex regional pain syndrome type I. J. Hand Surg. 2012;37(2):288–296. doi: 10.1016/j.jhsa.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Maihofner C., Handwerker H.O., Neundorfer B., Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63(4):693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- 37.Pleger B., Tegenthoff M., Ragert P., Forster A.F., Dinse H.R., Schwenkreis P. Sensorimotor retuning [corrected] in complex regional pain syndrome parallels pain reduction. Ann. Neurol. 2005;57(3):425–429. doi: 10.1002/ana.20394. [DOI] [PubMed] [Google Scholar]
- 38.De Rooij A.M., de Mos M., van Hilten J.J., Sturkenboom M.C., Gosso M.F., van den Maagdenberg A.M. Increased risk of complex regional pain syndrome in siblings of patients? J. Pain. 2009;10(12):1250–1255. doi: 10.1016/j.jpain.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Higashimoto T., Baldwin E.E., Gold J.I., Boles R.G. Reflex sympathetic dystrophy: complex regional pain syndrome type I in children with mitochondrial disease and maternal inheritance. Arch. Dis. Child. 2008;93(5):390–397. doi: 10.1136/adc.2007.123661. [DOI] [PubMed] [Google Scholar]
- 40.Puchalski P., Zyluk A. Complex regional pain syndrome type 1 after fractures of the distal radius: a prospective study of the role of psychological factors. J. Hand Surg. 2005;30(6):574–580. doi: 10.1016/j.jhsb.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Mekhail N., Kapural L. Complex regional pain syndrome type I in cancer patients. Curr. Rev. Pain. 2000;4(3):227–233. doi: 10.1007/s11916-000-0084-5. [DOI] [PubMed] [Google Scholar]
- 42.Bazrafshan S., Pacheco M., Ortiz J.C. Underlying adenocarcinoma of the lung metastasizing to the proximal phalanx of the foot causing complex regional pain syndrome: a case report. J. Am. Podiatr. Med. Assoc. 2017;107(2):150–154. doi: 10.7547/15-179. [DOI] [PubMed] [Google Scholar]
- 43.Guthmiller K.B., Varacallo M. StatPearls [Internet] StatPearls Publishing; 2018. Pain, complex regional pain syndrome (Reflex Sympathetic Dystrophy, RSD, CRPS) [Google Scholar]
- 44.O'Connell N.E., Wand B.M., McCauley J., Marston L., Moseley G.L. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst. Rev. 2013;4:CD009416. doi: 10.1002/14651858.CD009416.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackey S., Feinberg S. Pharmacologic therapies for complex regional pain syndrome. Curr. Pain Headache Rep. 2007;11(1):38–43. doi: 10.1007/s11916-007-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goh E.L., Chidambaram S., Ma D. Complex regional pain syndrome: a recent update. Burns Traumaatol. 2017;5(1):2. doi: 10.1186/s41038-016-0066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birklein F., Dimova V. Complex regional pain syndrome–up-to-date. Pain Rep. 2017;2(6) doi: 10.1097/PR9.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins C.K. Physical therapy management of complex regional pain syndrome I in a 14-year-old patient using strain counterstrain: a case report. J. Man. Manip. Ther. 2007;15(1):25–41. doi: 10.1179/106698107791090150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazaro R.P. Complex regional pain syndrome: medical and legal ramifications of clinical variability and experience and perspective of a practicing clinician. J. Pain Res. 2017;10:9. doi: 10.2147/JPR.S119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karmarkar A., Lieberman I. Mirror box therapy for complex regional pain syndrome. Anaesthesia. 2006;61(4):412–413. doi: 10.1111/j.1365-2044.2006.04605.x. [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen-Barr E., Held U., Grooten W.J. Non-steroidal anti-inflammatory drugs for sciatica: an updated Cochrane review. Spine. 2017;42:586e94. doi: 10.1097/BRS.0000000000002092. [DOI] [PubMed] [Google Scholar]
- 52.Breuer A.J., Mainka T., Hansel N., Maier C., Krumova E.K. Short-term treatment with parecoxib for complex regional pain syndrome: a randomized, placebo controlled double-blind trial. Pain Physician. 2014;17:127–137. [PubMed] [Google Scholar]
- 53.Eckmann M.S., Ramamurthy S., Griffin J.G. Intravenous regional ketorolac and lidocaine in the treatment of complex regional pain syndrome of the lower extremity: a randomized, double-blinded, crossover study. Clin. J. Pain. 2011;27:203–206. doi: 10.1097/AJP.0b013e3181fd5150. [DOI] [PubMed] [Google Scholar]
- 54.Shim H., Rose J., Halle S., Shekane P. Complex regional pain syndrome: a narrative review for the practicing clinician. Br. J. Anaesth. 2019 doi: 10.1016/j.bja.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varenna M., Adami S., Sinigaglia L. Bisphosphonates in Complex Regional Pain syndrome type I: how do they work? Clin. Exp. Rheumatol. 2014;32:451–454. [PubMed] [Google Scholar]
- 56.Chevreau M., Romand X., Gaudin P., Juvin R., Baillet A. Bisphosphonates for treatment of Complex Regional Pain Syndrome type 1: a systematic literature review and meta-analysis of randomized controlled trials versus placebo. Jt. Bone Spine. 2017;84:393–399. doi: 10.1016/j.jbspin.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Dahan A., Olofsen E., Sigtermans M., Noppers I. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur. J. Pain. 2011;15(3):258–267. doi: 10.1016/j.ejpain.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Correll G.E., Maleki J., Gracely E.J., Muir J.J., Harbut R.E. Subanesthetic keta¬mine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5(3):263–275. doi: 10.1111/j.1526-4637.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg M.E., Domsky R., Scaringe D. Multi-day low dose ketamine infusion for the treatment of CRPS. Pain Physician. 2005;8:175–179. [PubMed] [Google Scholar]
- 60.Dworkin R.H., O’Connor A.B., Backonja M. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 61.Serpell M.G., Neuropathic pain study group Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557–566. doi: 10.1016/S0304-3959(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 62.van de Vusse A.C., Stomp-van den Berg S.G., Kessels A.H., Weber W.E. Randomised controlled trial of gabapentin in complex regional pain syndrome type 1 [ISRCTN84121379] BMC Neurol. 2004;4:13. doi: 10.1186/1471-2377-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown S., Johnston B., Amaria K. A randomized controlled trial of amitriptyline versus gabapentin for complex regional pain syndrome type I and neuropathic pain in children. Scand. J. Pain. 2016;13:156e63. doi: 10.1016/j.sjpain.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 64.Goebel A. Immunoglobulin responsive chronic pain. J. Clin. Immunol. 2010;(suppl 1):S103–S108. doi: 10.1007/s10875-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 65.Goebel A., Blaes F. Complex regional pain syndrome, prototype of a novel kind of autoimmune disease. Autoimmun. Rev. 2013;12(6):682–686. doi: 10.1016/j.autrev.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Fischer S.G.L., Collins S., Boogaard S., Loer S.A., Zuurmond W.W.A., Perez R.S.G.M. Intravenous magnesium for chronic complex regional pain syndrome type 1 (CRPS-1) Pain Med. 2013;14(9):1388–1399. doi: 10.1111/pme.12211. [DOI] [PubMed] [Google Scholar]
- 67.Howard B.A., Roy L., Kaye A.D., Pyati S. Utility of radionuclide bone scintigraphy in complex regional pain syndrome. Curr. Pain Headache Rep. 2018;22(1):7. doi: 10.1007/s11916-018-0659-7. [DOI] [PubMed] [Google Scholar]
- 68.Duong S., Bravo D., Todd K.J., Finlayson R.J., Tran D.Q. Treatment of complex regional pain syndrome: an updated systematic review and narrative synthesis. Can. J. Anesthesia/Journal canadien d'anesthésie. 2018;65(6):658–684. doi: 10.1007/s12630-018-1091-5. [DOI] [PubMed] [Google Scholar]
- 69.Russo M.A., Santarelli D.M. A novel compound analgesic cream (Ketamine, Pentoxifylline, Clonidine, DMSO) for complex regional pain syndrome patients. Pain Pract. 2016;16(1):E14–E20. doi: 10.1111/papr.12404. [DOI] [PubMed] [Google Scholar]
- 70.Van Buyten J.P., Smet I., Liem L., Russo M., Huygen F. Stimulation of Dorsal Root Ganglia for the Management of Complex Regional Pain Syndrome: a Prospective Case Series. Pain Pract. 2015;15:208–216. doi: 10.1111/papr.12170. [DOI] [PubMed] [Google Scholar]
- 71.Kemler M.A., de Vet H.C., Barendse G.A., van den Wildenberg F.A., van Kleef M. Effect of spinal cord stimulation for chronic complex regional painsyndrome type I: five-year final follow-up of patients in a randomizedcontrolled trial. J. Neurosurg. 2008;108(2):292–298. doi: 10.3171/JNS/2008/108/2/0292. [DOI] [PubMed] [Google Scholar]
- 72.Goto S., Taira T., Horisawa S., Yokote A., Sasaki T., Okada Y. Spinal cord stimulation and intrathecal baclofen therapy: combined neuromodulation for treatment of advanced complex regional pain syndrome. Stereotact. Funct. Neurosurg. 2013;91(6):386–391. doi: 10.1159/000350022. [DOI] [PubMed] [Google Scholar]
- 73.Rocco A.G., Kaul A.F., Reisman R.M. A comparison of regional intravenous guanethidine and reserpine in reflex sympathetic dystrophy. A controlled, randomized, double-blind crossover study. Clin. J. Pain. 1989;5:205–209. doi: 10.1097/00002508-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Aradillas E., Schwartzman R.J., Grothusen J.R., Goebel A., Alexander G.M. Plasma exchange therapy in patients with complex regional pain syndrome. Pain Physician. 2015;18(4):383–394. [PubMed] [Google Scholar]
- 75.Zollinger P.E., Tuinebreijer W.E., Breederveld R.S., Kreis R.W. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J. Bone Joint Surg. 2007;89(7):1424–1431. doi: 10.2106/JBJS.F.01147. [DOI] [PubMed] [Google Scholar]
- 76.Zollinger P.E., Ünal H., Ellis M.L., Tuinebreijer W.E. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop. J. 2010;4:62. doi: 10.2174/1874325001004020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez R.S., Zuurmond W.W., Bezemer P.D., Kuik D.J., van Loenen A.C., de Lange J.J. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2013;102(3):297–307. doi: 10.1016/S0304-3959(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 78.Langendijk P.N., Zuurmond W.W., van Apeldoorn H.A., van Loenen A.C., de Lange J.J. Good results of treatment of reflex sympathetic dystrophy with a 50% dimethylsulfoxide cream. Ned. Tijdschr. Geneeskd. 1993;137(10):500–503. [PubMed] [Google Scholar]
- 79.Robinson J. The treatment of complex regional pain syndrome type 1. Australas. Musculoskeletal Med. 2002;7(2):101. [Google Scholar]
- 80.Younger J., Parkitny L., McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin. Rheumatol. 2014;33(4):451–459. doi: 10.1007/s10067-014-2517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chopra P., Cooper M.S. Treatment of complex regional pain syndrome (CRPS) using low dose naltrexone (LDN) J. Neuroimmune Pharmacol. 2013;8(3):470–476. doi: 10.1007/s11481-013-9451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams K., Guarino A., Raja S.N. fourth ed. 2018. Complex regional pain syndrome; pp. 223–232. (Essentials of Pain Medicine). [Google Scholar]
- 83.Harden R.N., Bruehl S., Perez R.S., Birklein F., Marinus J., Maihofner C., Lubenow T., Buvanendran A., Mackey S., Graciosa J., Mogilevski M. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150(2):268–274. doi: 10.1016/j.pain.2010.04.030. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner-Stokes L. Reflex sympathetic dystrophy – a complex regional pain syndrome. Disabil. Rehabil. 2002;24:939–947. doi: 10.1080/0963828021000007950. [DOI] [PubMed] [Google Scholar]
- 85.Muir J.M., Vernon H. Complex regional pain syndrome and chiropractic. J. Manipulative Physiol. Therapeut. 2000;23(7):490–497. doi: 10.1067/mmt.2000.108816. [DOI] [PubMed] [Google Scholar]
- 86.Goebel A., Barker C., Birklein F., Brunner F., Casale R., Eccleston C., Perrot S. Standards for the diagnosis and management of complex regional pain syndrome: results of a European pain Federation task force. Eur. J. Pain. 2019 doi: 10.1002/ejp.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wasner G., Schattschneider J., Binder A., Baron R. Complex regional pain syndrome – diagnostic, mechanisms, CNS involvement and therapy. Spinal Cord. 2003;41:61–75. doi: 10.1038/sj.sc.3101404. [DOI] [PubMed] [Google Scholar]
- 88.Birklein F., Ajit S.K., Goebel A., Perez R.S., Sommer C. Complex regional pain syndrome—phenotypic characteristics and potential biomarkers. Nat. Rev. Neurol. 2018;14(5):272. doi: 10.1038/nrneurol.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnhoorn K.J., van de Meent H., van Dongen R.T.M., Klomp F.P., Groenewoud H., Samwel H., Nijhuis-van der Sanden M.W.G., Frölke J.P.M., Staal J.B. Pain exposure physical therapy (PEPT) compared to conventional treatment in complex regional pain syndrome type 1: a randomised controlled trial. BMJ Open. 2015;5(12):1–11. doi: 10.1136/bmjopen-2015-008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaynberg E.A., Sakellariou A. Carpal Tunnel Syndrome and Related Median Neuropathies. Springer; Cham: 2017. Complex regional pain syndrome and Carpal Tunnel surgery; pp. 297–300. [Google Scholar]
- 91.Casale R., Atzeni F., Sarzi-Puttini P. The therapeutic approach to complex regional pain syndrome: light and shade. Clin. Exp. Rheumatol. 2015;33(1 Suppl 88):S126–S139. [PubMed] [Google Scholar]
- 92.Brun C., Giorgi N., Pinard A.M., Gagné M., McCabe C.S., Mercier C. Exploring the relationships between altered body perception, limb position sense, and limb movement sense in complex regional pain syndrome. J. Pain. 2019;20(1):17–27. doi: 10.1016/j.jpain.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 93.Smart K.M., Wand B.M., O’Connell N.E. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst. Rev. 2016;2:CD010853. doi: 10.1002/14651858.CD010853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bortolotto J. Reflex sympathetic dystrophy: an enigmatic improvement with spinal manipulation. J. Can. Chiropr. Assoc. 2000;44(4):245. [Google Scholar]
- 95.Vacariu G. Complex regional pain syndrome. Disabil. Rehabil. 2002;24(8):435–442. doi: 10.1080/09638280110108869. [DOI] [PubMed] [Google Scholar]